What are the options for myocardial reperfusion?
It is known that the process of complete or partial restoration of blood flow in the ischemic zone of the myocardium can occur spontaneously or artificially [2, 3, 7].
Spontaneous reperfusion can develop due to lysis or recanalization of a coronary thrombus, cessation of coronary artery spasm, or increased collateral blood flow in the ischemic area.
Artificial reperfusion is achieved using intracoronary or intravenous administration of thrombolytic agents, as well as surgical methods (percutaneous transluminal coronary angioplasty (PTCA), coronary artery bypass grafting, etc.) [5, 25, 39, 49]. However, despite the differentiated mechanism, the resumption of blood flow in the occluded artery causes a number of processes, combined under the term “myocardial reperfusion injury,” which negatively affect the restoration of the function of the ischemic myocardium [1, 3, 9, 43].
What are the known manifestations of myocardial reperfusion injury?
Myocardial reperfusion injury can manifest itself as:
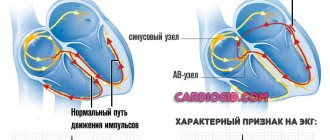
— reperfusion arrhythmias (RA), which are represented by ventricular extrasystole, accelerated idioventricular rhythm (AVR), ventricular tachycardia (VT) and ventricular fibrillation (VF) [14, 26, 28, 32, 53];
— the phenomenon of “stunning” of the myocardium (stunning myocardium), that is, its reversible post-ischemic dysfunction [12, 17, 44, 48];
— damage to microvasculature vessels and lack of restoration of coronary blood flow at the tissue level (no-reflow phenomenon) [31, 33, 47];
— accelerated development of necrosis of cardiomyocytes (CMC), the function of which was impaired by previous ischemia [1, 43].
The greatest problem is RA, since the mechanisms of their occurrence and the need for their treatment have not yet been clarified.
Reperfusion-induced arrhythmias usually occur immediately after restoration of vessel patency as a consequence of acute cellular, metabolic and local electrophysiological changes [2, 3, 42, 51].
What are the known mechanisms of myocardial reperfusion injury?
The development of myocardial reperfusion injury, and therefore RA, is based on the following mechanisms, which complement each other and are interrelated:
— adverse effects of reoxygenation of ischemic tissue with the formation of free oxygen radicals (“oxygen paradox”) [1, 8, 11, 15];
— excessive intake of calcium ions (Ca2+) from the extracellular space into the CMC with subsequent dysfunction of mitochondria, decreased production of adenosine triphosphate (ATP), the formation of contracture of cardiomyocytes and their subsequent death (“calcium paradox”) [12, 18, 51];
— mechanical damage to the CMC during restoration of blood flow [8, 22, 43].
The positive effect of oxygen on the functional state of the heart in the early period of MI (myocardial infarction) is largely due to a decrease in the size of the ischemic zone and the preservation of a larger number of CMCs capable of effective contraction [6, 9, 15, 35]. On the other hand, reoxygenation is an important factor contributing to the development of arrhythmias [2, 31, 36, 47].
With the formation of hydroxyl radical (OH–), which is a strong oxidizing agent and triggers the processes of lipid peroxidation (LPO) in the biomembranes of cardiomyocytes [2, 5, 8, 15], it was found that under its influence the cause of bradyarrhythmias and subsequent cardiac arrest is not damage to the contractile myocardium and asystole, and damage to the sinus node and depression of the generation of impulses in it, that is, dysfunction of automaticity [15].
Electrophysiological studies have established that an increase in the level of free radicals leads to arrhythmogenic changes in the characteristics of the action potential, disturbances in various phases of depolarization and an increase in automaticity, which manifests itself in the form of rhythm disturbances [10, 14, 15, 41].
Much attention in the development of reperfusion injury is given to activated polymorphonuclear leukocytes, which are capable of producing large amounts of superoxide anions and are a source of proteinases, in particular elastase, collagenase and lipoxygenase, secreted into the extracellular environment during degranulation and having a powerful alterative effect on cell membranes [1, 8, 9, 29, 48]. They also promote the release of biologically active substances (thromboxane, leukotrienes, platelet-activating factor), which are involved in the local inflammatory reaction [15, 31]. In addition, the importance of neutrophils in the pathogenesis of myocardial reperfusion injury is due to their ability to clog capillaries in the ischemia/reperfusion zone, which underlies the no-reflow phenomenon [21, 24, 33].
An equally significant mechanism of myocardial reperfusion injury is early cell overload with calcium [18, 20, 22, 46]. The calcium content in the cytoplasm of CMC increases even during the period of ischemia due to dysfunction of the K+/Na+-Ca2+ pumps and the Na+/Ca2+ exchange mechanism under conditions of ATP deficiency. Immediately after reperfusion, ATP resynthesis occurs, which makes it possible to resume the reuptake of Ca2+ by the sarcoplasmic reticulum and its “pumping” into the extracellular environment. Since by this time the concentration of Ca2+ in the cytoplasm of cells is significantly increased, the amplitude of calcium currents increases sharply, which creates conditions for the occurrence of RA [1, 9, 14, 51].
During ischemia/reperfusion due to excess oxygen free radicals, damage to the microvasculature is observed in many organs, including the heart, which leads to the occurrence of the no-reflow phenomenon [10, 24, 33]. According to a number of authors, during restoration of blood flow, a decrease in coronary perfusion leads to the occurrence of arrhythmias and post-ischemic contractile dysfunction (stunning myocardium) [12, 17, 44]. This can be confirmed by the fact that when using free radical scavengers and vasodilators (sodium nitroprusside, dipyridamole) [15, 23], there is no increase in perfusion pressure in the coronary arteries, a rapid restoration of the action potential is observed, which helps restore myocardial contractility and reduce the incidence of arrhythmias [10, 14, 42].
There is an opinion about the action of an additional (mechanical) factor of damage to the CMC membranes at the initial stage of blood flow restoration due to their overstretching and ruptures [8, 9, 19, 22].
The above changes, along with ischemia and cell necrosis due to AMI, contribute to the formation of an arrhythmogenic substrate, which, according to modern concepts, is a zone of electrical instability and is characterized by a disruption of the normal physiological relationships between the speed of impulse conduction and the duration of the refractory period in neighboring groups of myocytes. This causes local disturbances in conductivity, refractoriness and automaticity, which leads to the formation of electrical inhomogeneity of the ventricular myocardium [3, 4, 14, 42].
The development of AMI is accompanied by significant activation of the sympathoadrenal system and the release of catecholamines, which are involved in the development of ischemia/reperfusion processes. Their effect leads to destabilization of lipid metabolism in the CMC, activating lipid peroxidation processes, disruption of the regulation of vascular tone, changing myocardial microcirculation and thereby participating in the development of the no-reflow phenomenon. In addition, catecholamines have a direct effect on the electrophysiological parameters of the myocardium, activating ectopic automatism and the trigger mechanism for initiating ventricular arrhythmias, and also contribute to the formation of a re-entry loop and the initiation of VT and VF [3, 4, 11, 42]. The above explains the possibility of using blockers of adrenergic structures to prevent the development of reperfusion complications [3, 42].
Reperfusion syndrome, methods of prevention and treatment of secondary tissue damage during ischemia
Relevance. Critical conditions, injuries, many diseases and the early postoperative period are often accompanied by ischemia of tissues and organs. Emerging circulatory disorders can manifest themselves with clinically significant symptoms (hypotension, tachycardia), and also occur without visible clinical manifestations. Most often, these disorders occur at the level of microcirculation, are fleeting and pass without a trace, however, in severe critical conditions, blood circulation is disrupted in the regional blood supply system, which leads to the development of organ or multiple organ failure. Treatment of patients during this period is extremely difficult and requires great effort and expense.
In clinical practice, the doctor quite often encounters various manifestations of tissue ischemia, which can be either short-term or long-term, local or widespread. Depending on this, the result of ischemia can be complete restoration of function and structure, or necrosis and anatomical deficit.
The main task of the clinician in these cases is to restore macro- and microcirculation. Meanwhile, the paradox of treatment is that when blood circulation is restored in ischemic tissues, oxygen delivery is accompanied by the formation of its active forms, which damage cell membranes [9, 15]. As a result, secondary damage to tissues and organs occurs and reperfusion syndrome develops. Good turns into harm. The severity of this syndrome is determined by the prevalence and duration of ischemia preceding the restoration of blood circulation.
Reperfusion syndrome is a complex of clinical manifestations of restoration of blood circulation in previously ischemic tissues, accompanied by damage to cells, tissues and organs at the local and systemic level with the development of multiple organ failure.
This syndrome is a universal response of the body to ischemia of any etiology.
Clinical conditions in which reperfusion syndrome develops
It has long been noted in medical practice that in some conditions, while hemodynamic parameters are improving, there is a deterioration in the general condition. It is a known fact that after a traumatic brain injury (TBI) and relative stabilization of hemodynamics with the start of treatment, the condition of the victims worsens, and the degree of depression of consciousness increases [2, 3, 5]. Neurosurgeons and resuscitators have long been looking for ways to prevent this secondary brain damage in severe TBI.
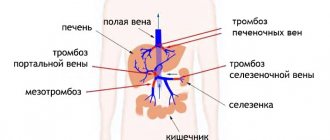
Inclusion syndrome has also been described in patients after reconstructive operations for occlusive lesions of the vessels of the lower extremities, when impaired renal function, coronary blood supply, and respiratory disorders such as acute respiratory distress syndrome (ARDS) occur [ 3]. The peak of these manifestations is observed one day after the start of treatment.
A clear clinical picture of inclusion syndrome was noted when perftoran was used in patients with Leriche syndrome. Improvement in peripheral circulation (warming of the extremities) was accompanied by severe lower back pain, increased levels of nitrogenous wastes and medium-weight molecules, acidosis, heart rhythm disturbances, a decrease in the oxygenation index, and the development of acute lung injury.
When restoring blood circulation in the coronary vessels during myocardial infarction after balloon angioplasty, stenting or thrombolysis, heart rhythm disturbances often occur that are difficult to treat, and effective methods for preventing these arrhythmias have not yet been proposed [4, 6, 9]. Reperfusion syndrome in experimental myocardial infarction was first described in 1960 [14]. The authors described signs of myocardial damage: cellular edema, myofibril contracture, sarcolemmal ruptures, and mitochondrial damage. Today, complications of operations to restore blood flow to the infarct - dependent artery are associated with reperfusion injuries of the myocardium [9, 10, 11, 13].
Severe combined injuries, extended and combined surgical interventions, massive blood loss, intoxication and other conditions are accompanied by centralization of blood circulation. An important component of the treatment of such patients is the restoration of blood circulation in the microcirculatory system. In this case, the main task is to deliver oxygen to tissues where there is not enough of it. The delivery of oxygen to ischemic tissues is accompanied by the development of an oxidative cascade. Serotonin plays an important role in restoring blood circulation in the microcirculatory system and regulating capillary blood flow [1, 7].
Significant tissue damage during the period of restoration of blood circulation is clinically accompanied by local and general disorders. Thus, locally, with brain reperfusion, edema increases, and neurological deficit increases clinically; pain appears in the operated limb, trophic disorders increase; changes in the heart lead to rhythm disturbances. A systemic manifestation of reperfusion syndrome is the development of multiple organ failure. Most often, the phenomena of ARDS, renal failure and encephalopathy increase.
Thus, reperfusion syndrome develops in the place where there was an episode of ischemia, followed by restoration of blood circulation and oxygen delivery [8]. The longer the duration and extent of ischemia, the more pronounced the symptoms of reperfusion.
Pathogenesis of reperfusion syndrome
In critical situations accompanied by circulatory disorders, large volumes of tissue suffer from hypoxia. The biochemical “storm” leads to a catastrophic increase in the corresponding ischemic markers and an increase in lactate levels. Acidic foods cause spasm of the precapillary sphincters. With ischemia lasting more than 2 hours , most cells die, anatomical structures suffer, and organ failure develops. This category of patients more often develops renal, respiratory and heart failure.
When the blood is shunted, metabolism takes the path of anaerobic glycolysis, and cell energy deficiency occurs. Acidic metabolic intermediate products accumulate. When blood circulation and oxygen delivery to tissues are restored, the oxidation process is activated, which leads to secondary damage to cell membranes by reactive oxygen radicals (Fig. 1). Their number is increasing exponentially.
During ischemia, ATP is converted to AMP, followed by the formation of adenosine, inosine, and hypoxanthine. The main production of tissue-damaging reactive oxygen species (ROS) occurs during reperfusion, when, in the presence of xanthine oxidase, oxygen converts hypoxanthine to urate and reactive radicals are formed. ROS destroy cell membranes, which leads to further deterioration of tissue [3, 5, 15]. This is how secondary tissue damage occurs (Fig. 2).
Treatment and prevention of reperfusion syndrome
The main goals of treatment for these conditions are aimed at restoring blood supply, oxygen delivery to tissues and perfusion in the microcirculation system. This is achieved by replenishing circulating blood volume (CBV), globular volume, reducing blood viscosity and improving microcirculation in areas of impaired circulation, through the use of various groups of drugs, including direct anticoagulants, peripheral vasodilators (mainly slow calcium channel blockers), pentoxifylline (trental) , serotonin, myotropic antispasmodics (papaverine), etc. [7, 10, 12]. Papaverine also has a therapeutic effect in an acidic environment and relieves vascular spasm even after removing a long-term tourniquet.
In recent years, the use of serotonin has been very promising, which, according to Doppler ultrasound, increases the volumetric systolic (Qas) and average (Qam) velocity of capillary blood flow up to 20 times, thereby reducing the transition zones of ischemia (Vrublevsky O. Yu. et al., 1997). This effect of serotonin allows it to be prescribed to patients with myocardial infarction, ischemic stroke, diabetic foot syndrome, and ARDS.
The better blood circulation in tissues is restored and oxygen delivery increases, the more ROS is formed and the more pronounced secondary damage becomes. To correct such conditions, there have been proposals to reduce the delivery of oxygen to damaged tissues, but this is not a way out of the current situation. Antioxidants have recently become widely used to neutralize ROS (Fig. 3). The most commonly used drug in this group is Mexidol, which significantly reduces the severity of oxidative stress. However, it should be understood that its effect is aimed at substrates that have already formed in the areas of ischemia elimination. Other antiradical agents are also used for the same purpose: superoxide dismutase preparations, vitamin E, Vitamin A, Vitamin C, etc.
Superoxide dismutase (SOD) is a catalyst for the reverse reaction - dismutation (reverse conversion) of ROS into oxygen and hydrogen peroxide. SOD works together with catalase, which breaks down H2O2 into molecular oxygen and water. In this regard, superoxide dismutase drugs (Orgotein, Rexod, etc.) should be included in the complex therapy for reperfusion syndrome.
Pathogenetically, the most effective treatment for reperfusion syndrome should be a drug that can prevent the formation of reactive oxygen radicals. This can prevent the formation of ROS and thereby secondary damage to cell membranes, which will prevent reperfusion syndrome.
These pharmacological properties characterize the drug allopurinol, which has the specific ability to inhibit the enzyme xanthine oxidase, which is involved in the conversion of hypoxanthine to xanthine. During this reaction, the process of active formation of ROS also starts [6, 15]. By inhibiting xanthine oxidase, allopurinol prevents the formation of reactive oxygen species, and, being essentially a pro-oxidant, protects tissues from chemically active influences. For this purpose, allopurinol tablets should be administered orally (parenteral forms of allopurinol are currently not available on the pharmaceutical market), through a gastric or intestinal tube after crushing, dissolving in water. The dose is 300 - 500 mg per day. Prescribed after restoration of intestinal absorption function. Allopurinol can also be prescribed orally 2 to 3 hours before major traumatic surgical interventions, before upcoming thrombolysis or balloon angioplasty, before restoring blood circulation in the extremities.
In the complex treatment of severe conditions, other antioxidants should be used, which significantly improve treatment results. Antioxidants extinguish the fire of oxidative stress, and allopurinol prevents it from flaring up.
Thus, the inclusion of allopurinol in the complex of intensive therapy for severe conditions will prevent reperfusion damage to cells and tissues, the development of organ and multiple organ failure. The use of allopurinol for the prevention of reperfusion syndrome should raise the effectiveness of intensive therapy to a higher level and provide a good clinical and economic effect.
Prevention of reperfusion syndrome eliminates the formation of ROS, ensuring the entry of O2 directly into the cell, restores the aerobic metabolic pathway and increases its energy value, which helps protect tissues and organs from secondary damage. Recurrent tissue damage from TBI can be prevented; the appearance of arrhythmias, sometimes fatal, after thrombolysis and angioplasty of the coronary vessels; development of multiple organ failure, acute renal failure, ARDS in severe concomitant injury; acute coronary syndrome when restoring blood circulation in the extremities with Leriche syndrome, diabetic foot.
Allopurinol as a prophylaxis for reperfusion syndrome should be used at the emergency stage before thrombolysis or angioplasty in patients with myocardial infarction, ischemic cerebral stroke, before surgery in patients with acute thrombosis, ischemia, tourniquet application, during extensive surgical operations with impending large blood loss. It should become a pathogenetically substantiated means of preventing secondary tissue damage in case of any significant tissue ischemia.
Bibliography
- Vrublevsky O. Yu., Ardashev V. N., Tyurin V. P. et al. Experience of thrombolytic therapy for myocardial infarction. //Military - honey magazine - 1997. - No. 11. - pp. 40-45.
- Hospital surgery. Syndromology: textbook Abdullaev A. G. et al.; edited by N. O. Milanova - 2013. - 440 p. [Hospital surgery. Sindromology: education guidance Abdullaev AG, etc. ; under the editorship of NO Milanov - 2013. – 440 pages].
- Zilber A.P. “Medicine of critical conditions” Book. 1. – 1995. pp. 174-176.
- Mashkovsky M.D. “Medicines” 16th edition. -2014. — 1216 p. .
- Neurology and neurosurgery, ed. A. N. Konovalova. Textbook. 2009. - 420 p. .
- "Recommendations for myocardial revascularization." – Russian Journal of Cardiology. – 2015. – No. 2. 81 p. ["Recommendations for myocardial revascularization". – Russian journal of cardiology. – 2015. – No. 2. 81 pages].
- Simonenkov A.P., Klyuzhev V.M. Serotonin deficiency syndrome - M.: BINOM. 2013. – 96 p. .
- Appleby MA et al. Angiographic assessment of myocardial perfusion: TIMI myocardial perfusion (TMP) grading system. //Heart. 2001. Vol. 86, No. 5. P. 485–486.
- Ganame J. et al. Impact of myocardial haemorrhage on left ventricular function and remodeling in patients with reperfused acute myocardial infarction. //Eur. Heart J. 2009. Vol. 30, No. 12. P. 1440–1449.
- Garcia-Dorado D. et al. Calcium-mediated cell death during myocardial
reperfusion. // Cardiovasc. Res. 2012. Vol. 94, No. 2. P. 168–180.
- Sivaraman V., Yellon DM Pharmacologic Therapy That Simulates Conditioning for Cardiac Ischemic/Reperfusion Injury // J. Cardiovasc. Pharmacol. Ther. 2014. Vol. 19, No. 1. P. 83–96.
- Ungi I. et al. Myocardial protection with enalaprilat in patients unresponsive to ischemic preconditioning during percutaneous coronary intervention. //Can. J. Physiol. Pharmacol. 2008. Vol. 86, No. 12. P. 827–834.
- Widimsky P. et al. Reperfusion therapy for ST elevation acute myocardial infarction in Europe: description of the current situation in 30 countries. //Eur. Heart J. 2010. Vol. 31, No. 8. P. 943–957.
- Jennings RB et al. Myocardial necrosis induced by temporary occlusion of a coronary artery in the dog. //Arch. Pathol. 1960. Vol. 70. P. 68–78.
- Zweier J., Talukder M. The role of oxidants and free radicals in reperfusion injury // Cardiovasc. Res. 2006. Vol. 70, No. 2. P. 181–190.
Does the development of reperfusion arrhythmias depend on the speed of reperfusion?
A relationship has been established between the rate of reperfusion, the duration of previous ischemia and the occurrence of reperfusion arrhythmias [3, 27, 37, 47]. The sudden restoration of blood flow leads to reoxygenation and washout of toxic metabolites; this imparts heterogeneity and electrical instability to the tissue, which, in turn, leads to the development of arrhythmias [3, 14, 24]. The duration of ischemia affects the severity of metabolic damage during the reperfusion period: the longer the period of myocardial ischemia, the greater the degree of ischemic/reperfusion damage and the higher the incidence of RA development [9, 25, 39].
Is there a relationship between reperfusion arrhythmias and previous ischemia?
There is a connection between the development of RA and the degree of reversible ischemia; it appears that RAs do not occur in necrotic cells [25, 43, 44]. This assumption explains the fact that energy (in the form of ATP) is required for the development of some RAs. It is believed that the peak occurrence of RA is between the 5th and 20th minutes after restoration of blood flow, and the decrease in the frequency of their occurrence is directly proportional to the depletion of ATP reserves [8, 43].
General manifestations of ischemic tissue reperfusion syndrome
General manifestations of reperfusion tissue damage are rare in plastic surgery due to the use of relatively small tissue complexes, the metabolic products of which are unable to significantly affect the vital functions of the entire organism. However, the author's personal experience indicates that in weakened patients who have undergone major surgery with significant blood loss, the threshold of sensitivity to toxemia is significantly reduced. Against this background, even small areas of dead tissue can become a source of severe and life-threatening intoxication.
It is all the more important to have an idea of the general reactions of the human body to local ischemic tissue damage, information about which is accumulated by each surgeon only during rare clinical observations. In total, three forms of general ischemic tissue reperfusion syndrome can be distinguished: fulminant, acute and chronic.
Fulminant form of tissue reperfusion syndrome. A life-threatening general reaction described as “replantation limb syndrome”, “replantation toxicosis”, occurs only in replantation surgery with late replantation (revascularization) of large segments of the limbs, as well as with the syndrome of their prolonged compression. The death of a significant amount of muscle and other tissue leads, with the restoration of blood circulation, to a powerful toxic effect on the body, the development of toxic shock and death of the patient within a few hours. That is why, at clearly critical periods of ischemia of large (and primarily muscle) masses, it is recommended to amputate the limb without restoring blood flow.
The acute form of tissue reperfusion syndrome is distinguished by the fact that the consequences of a metabolic catastrophe develop in the affected tissues on a smaller scale, and the disturbances in general hemodynamics are short-term, not so deep, and can be stopped through intensive therapy. However, the formation and leaching of a large amount of myoglobin into the general vascular bed leads to kidney blockade, which, in turn, can lead to the death of the patient from anuria against the background of toxemia.
With this form of reperfusion, foci of necrosis appear on the limb within a few hours, the true extent of which can be masked by the well-supplied skin. In the mildest cases, the use of hemodialysis in combination with local necrectomy can save both the patient's life and the limb. At the same time, the high risk (for the patient’s life) of such tactics is, as a rule, not justified due to severe subsequent dysfunction of the preserved segment.
In most situations, the patient can be saved only through urgent amputation of the affected tissue areas and intensive treatment using an artificial kidney.
The chronic form of tissue reperfusion syndrome occurs when a relatively small mass of tissue (mainly muscle) dies during replantation (revascularization) at the level of the lower leg or elbow joint. An important feature of the chronic form of tissue reperfusion syndrome is that the general reaction to the inclusion of tissues in the bloodstream is absent or not expressed in most cases. The blood supply to the skin distalpes of the damage level remains at a normal or slightly reduced level, despite the formation in some cases of small areas of necrosis.
At the same time, a significant part of the muscles dies, which is manifested by swelling of the limb, the presence of ischemic pain and the phenomena of general intoxication (hyperthermia, tachycardia, increasing anemia, development of immunodeficiency, etc.), occurring without significant impairment of the excretory function of the kidneys. In such situations, the desire to preserve a limb (often unpromising from the point of view of restoring function) can, due to the sharp weakening of the patient’s body, lead, despite intensive treatment, to catastrophic consequences.
In most cases, the way out is amputation of the affected segment or excision of non-viable tissue, carried out in the early stages.
Patient K., 30 years old, was transferred to the clinic from a city hospital 2 weeks (!) after a through gunshot wound to the left leg in the upper third with a perforated fracture of the proximal metaepiphysis of the tibia. According to the attached documentation, upon initial admission to the hospital, blood circulation in the limb was not impaired. During the treatment of the wound, the surgeons on duty performed its dissection and drainage on the posterior surface of the segment. Subsequently (after 7-10 days), signs of peripheral circulatory disorders appeared.
Upon admission to the clinic: the temperature of the limb is slightly reduced, but signs of satisfactory blood circulation in the skin are clearly visible in all parts of the lower leg and foot, with the exception of areas of local necrosis along the back surface of the heel and in the area of the IV-V fingers. There is no pulsation of the great vessels. The sensitivity of the skin below the middle of the lower leg is lost. Active movements of the foot are impossible. The general condition of the patient is satisfactory. Body temperature 38.2 C, pulse 104 beats/min, blood pressure 120/80 mm Hg. Art. The pain syndrome is not expressed. Angiography was not performed.
Intensive treatment was started (general detoxication therapy, vasoplegic drugs, MBT, blood transfusions), as a result of which the patient’s condition noticeably worsened. The range of morning and evening temperatures increased, the pain intensified, and anemia increased. Another week later (only 3 weeks after the injury), the oxygen tension in the skin of the middle third of the leg was 70 - 75% of normal. All this made it possible to evaluate the deterioration of the patient’s condition as a result of increased toxemia in response to an increase in collateral circulation. This conclusion became the basis for the operation. During the audit of the osteofascial sheaths, total necrosis of all muscles of the leg was discovered, and therefore amputation of the limb was carried out. A study of the drug revealed thrombosis of the popliteal artery in the trifurcation zone.
After the intervention, the patient's condition quickly improved.
It should be noted that when transplanting skin-muscle flaps, the death of significant muscle masses can also lead to the development of chronic ischemic tissue reperfusion syndrome. In one of our observations, a simultaneous lengthening of the short stump of the forearm was performed by transplanting two tissue complexes: the fibula with a skin-fascial flap and a thoracodorsal flap. The development of venous thrombosis on the 1st day after surgery and two failed attempts to restore venous outflow led over the course of another day to the fact that, although there was inflow to both flaps, the venous outflow was disrupted.
This led to the slow death of the transplanted tissues and the appearance of signs of severe intoxication, which did not decrease despite powerful therapy. Within a few hours, the patient developed an infectious septic condition with a real threat to life. Only removal of all transplanted tissues allowed the patient's condition to improve, although in a retrospective assessment this decision could be considered belated.
How do interventions influence the development of reperfusion arrhythmias?
The embolization of distal parts observed during interventional procedures cannot but play a role in the development of reperfusion arrhythmias. Stenting has a particular impact on the development of clinically significant embolization, more pronounced than with angioplasty [8]. According to J. Henriques et al. [31], who analyzed the results of primary angioplasty in 178 patients with AMI, the frequency of detection of signs of significant embolization (according to angiography with the formation of distal contrast filling defects) was 15.2%, and in 73% of patients good epicardial blood flow was recorded (TIMI class 3). The development of embolization was more often recorded in patients with anterior localization of the infarction, against the background of repeated infarction, and multivessel lesions. At 72 hours after the procedure, the final myocardial infarction size, measured by serial lactate dehydrogenase analysis, in patients with embolization was 2 times the size of the infarction in patients without embolization. The results of the 5-year follow-up were also expected: 44% of patients died during this period with the development of embolization and 9% without it (P < 0.001).
The authors who studied the significance of reperfusion arrhythmias occurring after successful recanalization of a closed coronary artery due to percutaneous percutaneous coronary angioplasty in patients with acute coronary syndrome with ST segment elevation indicated that the occurrence of RA immediately after PTCA indicates a favorable prognosis in this category of patients [32] . In 688 patients, of whom 22% were women, the mean age was 61 ± 14 years, diabetes mellitus occurred in 25%, dyslipidemia in 55%, arterial hypertension in 43%, and 41% of patients were smokers. RA was reported in 200 patients, or 29%. Patients with RA were less likely to have diabetes (16 vs. 30%, P <0.01) and hypertension (48 vs. 62%, P <0.01), and they had a shorter mean pain-to-balloon time ( 201 versus 234 minutes, P <0.01) than in patients without RA. Thirty-day mortality was 3.7% and 8.3% for patients with and without RA, respectively (P = 0.04). The relative risk of mortality for patients with RA was 0.46 (95% confidence interval 0.23–0.92 ) for 466 days.
Medical Internet conferences
Purpose of the study: to determine the possibilities of therapy for reperfusion ischemia in a patient with ST-segment elevation myocardial infarction after coronary stenting. Methods and materials: clinical observation. Results: Patient X., 49 years old, was admitted to the intensive care unit with a diagnosis of coronary artery disease. Acute coronary syndrome with ST segment elevation." At the prehospital stage, thrombolysis was performed with streptokinase 1.5 million units. The clinic performed coronary angiography: acute occlusion of the proximal segment of the anterior descending artery (ADA) was determined. Balloon angioplasty and LAD stenting were performed. After percutaneous coronary intervention (PCI), the patient’s condition is stable, there is no coronary pain, heart rate is 68 per minute, blood pressure is 120 and 80 mm Hg. Troponins, positive myoglobin, CPK 1770 U/l, CPK-MB 496 U/l, ECG: pathological Q wave, ST segment elevation in V2-V6, in dynamics a decrease in the ST segment and the formation of a negative T wave in V2-V6. According to echocardiography: LV FI 34%, hypokinesia of the anterior and lateral walls, interventricular septum, and apex. Treatment: acetylsalicylic acid 100 mg/day, clopidogrel 75 mg/day, heparin 1000 units/hour with infusamate, rosuvastatin 40 mg/day, metoprolol succinate 25 mg/day, perindopril 2.5 mg/day, isosorbide dinitrate 40 mg/day , spironolactone 50 mg/day. 5 hours after PCI, intense pain appeared in the heart area, weakening but not relieved after administration of nitrates and morphine 1% 1.0 ml. The ECG shows negative dynamics in the form of increased ST segment elevation in leads V2-V6. Based on the results of echocardiography, a further decrease in LVEF was determined to 28%. Troponins, myoglobin are positive, CPK 768 U/l, CPK-MB 50.9 U/l. This patient’s condition was assessed as reperfusion injury of the myocardium after LAD stenting. One mechanism of this damage is vasospasm. In this regard, a calcium channel blocker (amlodipine 2.5 mg/day) was added to treatment, and coronary pain was relieved.
Conclusions. Restoration of coronary blood flow as a result of stenting of the coronary arteries can create conditions for the occurrence of reperfusion myocardial ischemia, the “no-reflow” phenomenon is a slowdown in blood flow, which is manifested by recurrence of coronary pain, deterioration of ECG parameters, and a decrease in LVEF. The addition of Ca channel blockers to standard therapy made it possible to stabilize the patient's condition.
Can reperfusion arrhythmias cause sudden coronary death?
There is evidence that reperfusion arrhythmias can cause sudden death in the acute period of myocardial infarction. A patient with AMI underwent successful coronary artery recanalization using PTCA. Within one minute after insertion and inflation of the balloon, an idioventricular rhythm developed, accompanied by a drop in hemodynamics. Sinus rhythm was immediately restored when the balloon was reinflated, and oxygenation was thus interrupted again. The authors suggest that reperfusion followed by blood reoxygenation is one of the primary factors of arrhythmogenesis [13].
Is it possible to prevent the development of reperfusion arrhythmias with drug therapy?
Data from experimental and clinical studies in recent years indicate the possibility of preventing reperfusion damage to the CMC through the use of drugs with membrane-protective properties (trimetazidine, magnesium sulfate, quercetin), which leads to limiting the area of necrosis, preventing dilatation of the left ventricular cavity, increasing the electrical stability of the myocardium and therefore, reducing the incidence of RA [1, 2, 9, 38].
Some authors point to the cardioprotective effect of angiotensin-converting enzyme (ACE) inhibitors [34, 40, 45]. Angiotensin II is one of the mediators of the occurrence of reperfusion injury and RA; it helps to shorten the refractory period and increase heart rate [30, 50]. The vasoconstrictor effect of angiotensin II creates an oxygen deficiency, which can lead to myocardial ischemia, which is a powerful arrhythmogenic factor. It has been established that angiotensin II enhances the flow of Ca2+ not only through L-type Ca2+ channels, but also promotes its release from intracellular stores through type 1 receptors for angiotensin II; in addition, it promotes the release of catecholamines, which also leads to Ca2 overload [18, 20]. The beneficial effects of ACE inhibitors are realized by blocking the production of angiotensin II and increasing the amount of bradykinin, which can stimulate the production of prostaglandins and nitric oxide [34, 40]. In addition, ACE inhibitors prevent the development of vasospasm and increased platelet aggregation, and are also a donor of sulfhydryl groups that bind free radicals [50]. Recently, the role of nitric oxide (NO) in ischemia/reperfusion injury has been studied, but the exact mechanisms of its protective effect on the myocardium have not yet been established. It is assumed that its main cardioprotective effects are realized by eliminating the Ca2+ overload of the CMC, reducing the contractility of the heart muscle and its oxygen consumption, antagonism of beta-adrenergic stimulation, antioxidant action, opening of K+-ATP channels, eliminating the phenomenon of no-reflow and leukocyte infiltration, reducing the release of cytokines [16, 44, 52].
Although Ca2+ is the main pathological factor in the occurrence of reperfusion injury and RA, the use of Ca2+ channel blockers has not found widespread use [3, 43, 51]. At the same time, the effectiveness of drugs such as magnesium sulfate, adenosine, dipyridamole has been proven, the main mechanism of action of which is realized by modulating the transmembrane flow of Ca2+ ions and eliminating calcium overload of the CMC [23, 38, 46].
Thus, the development of RA due to myocardial reperfusion injury is a phenomenon that has a deep biochemical and neurohumoral basis. Studying the subtle electrophysiological and metabolic mechanisms of the occurrence of RA is necessary both to optimize the timing and method of restoring coronary blood flow, preventing the development of life-threatening arrhythmias, and influencing the key pathogenetic links of reperfusion damage.
The above data indicate an urgent need to develop new approaches to systemic and intracoronary thrombolysis, as well as surgical revascularization, which can significantly reduce the risk of reperfusion injuries.
Etiopathogenesis of reperfusion injuries in vascular surgery of the lower extremities
Oborin Alexander Andreevich
State budgetary healthcare institution of the Perm region "Order of the Badge of Honor" Perm Regional Clinical Hospital", Department of Cardiovascular Surgery, Perm, Russia Contacts:
Abstract . The presented review covers the topic of damaging tissue reoxygenation during lower limb revascularization. Possible mechanisms of occurrence are considered: from cellular and molecular bases to clinical manifestations, as well as the behavior of hemodynamics at the level of micro- and macrocirculation. Reperfusion syndrome is a mandatory phenomenon when restoring blood flow after prolonged ischemia, but there is a question about the severity of this condition. In addition, the question of prevention and correction arises. Many doctors in general surgical and specialized departments are forced to deal with acute limb ischemia and, as a consequence, the development of damaging reoxygenation during successful recanalization. Irreversible cell death of the tissue develops. Unfortunately, it is not always possible to save a limb in such a situation. The challenge is to find the optimal angioprotector, since the main post-occlusion changes occur precisely in the vessel wall. These and other issues are discussed in detail in the presented materials.
KEY WORDS : lower extremity vascular surgery, reperfusion injury, revascularization, reoxygenation.
Introduction
Back in 1885, when attempting to perform one of the first embolectomies from the femoral artery, the Russian surgeon F. Sabaneev proposed, in addition to embolectomy (to prevent the entry of toxic products from ischemic tissues into the body), to wash the vascular bed of the ischemic limb with physiological solution through the femoral vein [1 ]. Reperfusion syndrome, or the “no-reflow” phenomenon (the phenomenon of unrestored blood flow), consists of aggravating the severity of ischemia after technically successful recanalization of the arterial segment and is characterized by the lack of adequate blood flow at the tissue level. It is worth noting that reperfusion syndrome is not so much an unforeseen complication as a programmable and integral condition after radical surgical interventions in this category of patients. The studied literature covers reperfusion syndrome after revascularization of the myocardium and brain in both chronic and acute ischemia [2]. However, this problem occurs after reconstructive operations on the arteries of the lower extremities, which dictates a more detailed study of the “no-reflow” phenomenon, the search for diagnostics and adequate treatment when damaging reperfusion occurs. A technically successfully performed intervention does not guarantee restoration of peripheral blood flow and preservation of the limb if the pre- and postoperative period is inadequately formed, where one of the main complications is reperfusion syndrome. In practical medicine, there is an urgent need to study indicators of cell and tissue damage for diagnostic purposes and assess the functional state of organ damage [3].
Contents of the review
FW Blaisdell, in his work on the pathophysiology of reperfusion, called damaging oxygenation as a set of complications following the restoration of arterial blood flow in previously ischemic organs and tissues. It is generally accepted that the development of reperfusion syndrome is largely determined by the initial state of regional microcirculation. L.P. Churilov reveals the damaging mechanisms of post-occlusive hyperemia, saying that previously starved cells greedily absorb oxygen, forming such an amount of peroxide compounds that antioxidant systems cannot cope with, lipid peroxidation sharply increases, which leads to direct damage to cell membranes and free radical necrobiosis [ 4]. The systemic effect of active metabolites of lipid peroxidation is determined by the ability of the latter to leave membranes due to their more pronounced hydrophilicity compared to the original lipids, and this makes lipid peroxidation products one of the important factors in the pathogenesis of generalized hemodynamic disorders in the body. Oxidized phospholipids have a pronounced vasoactive and cardiotropic effect. During the reperfusion period, LPO products have a vasoconstrictive effect on organ and tissue vessels exposed to ischemic effects, causing a systemic hypotensive effect due to negative inotropic and cardiodepressive effects [5]. The damaging effects of ischemia do not become apparent until oxygenated blood reaches the organ [6]. This fact allows some authors to call ischemia and subsequent reperfusion a “two-hit” injury [7]. The development of cardiovascular, pulmonary, and renal failure of varying severity is a clinical manifestation of reperfusion damage to organs and tissues after surgery on the abdominal aorta [8]. The main destructive factor for a cell when blood flow is stopped is hypoxic necrobiosis. Necrobiosis is the process of cell death, a deep, partially irreversible stage of cell damage, immediately preceding its death. The essence is the loss of the cell's ability to produce ATP, loss of potassium, decrease in cytoplasmic pH, decrease in the activity of enzymatic systems, and saturation of the cell with calcium and sodium. A decrease in pH leads to inhibition of phosphofructokinase (reduction of anaerobic breakdown), as well as denaturation of some proteins [9]. And excess calcium concentration is toxic to the cell. Depletion of ATP reserves and a simultaneous increase in the concentration of lactic acid causes depolarization of membranes with disruption of the transport of substances through it due to the developing dysfunction of ion pumps. Potassium leaves the cell, causing an increase in the volume of extracellular fluid and the development of ischemic edema of the organ, and sodium and calcium enter it, resulting in an increase in intracellular volume with damage to intracellular structures, primarily energetically significant mitochondria [10].
As mentioned earlier, in bloodless tissues a large amount of purine bases is formed (since the cell, in conditions of lack of oxygen, switches to anaerobic nutrition), which, in turn, under conditions of a sharp improvement in blood supply, are the main suppliers of free radical molecules. At the same time, the activity of superoxide dismutase, catalase and glutathione reductase, responsible for neutralizing free radicals, is suppressed due to the effects of ischemia [11]. Reactive oxygen species, interacting with proteins and carbohydrates, cause disruption of the processes of methylation, oxidative deamination and lead to the formation of a number of substances toxic to the body: peroxides, hydroperoxides, ketones, aldehydes, etc. Interacting with cell membranes, free radical oxygen species induce peroxidation of membrane lipids , a change in the structure of their proteins with impaired permeability, further suppressing bioenergetic processes in the cell. An additional source of free radicals can be the overproduction of nitric oxide (NO is a reactive oxygen-containing radical) due to stimulation of NOS (an enzyme directly involved in the formation of nitric oxide) under hypoxic conditions. The release of large amounts of free radicals leads to endothelial damage and microcirculation block, which is associated with endothelial edema and an increase in the proportion of arteriolo-venular shunting. A decrease in the intensity of microcirculation and an increase in arteriovenous discharge are the basis of an important compensatory mechanism triggered in response to reperfusion injury. These changes in peripheral macrohemodynamics arise due to insufficient restoration of the function of the microcirculatory system, which is not able to cope with a sharp increase in arterial blood flow. This makes it possible to reduce further damage to the tissues of the ischemic organ, as well as the manifestations of autointoxication [12].
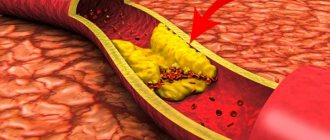
According to the authors, the main contribution to the development of reperfusion syndrome is made by leukocytes accumulating in the ischemic zone: polymorphonuclear leukocytes are capable of releasing free radicals with subsequent damage to the endothelium, proteolytic enzymes, including matrix metalloproteinases, leukotrienes, which stimulate platelets and endothelium. Endothelial dysfunction progresses. In the microvasculature, the action of superoxide promotes the degradation of hyaluronic acid in the endothelial collagen layer and basement membrane, which leads to microthrombosis and increased permeability of the vascular wall and causes the development of interstitial edema. In turn, the transition of fluid into the interstitial space leads to blood thickening, slowing blood flow and the formation of blood clots. Kryzhanovskaya S.A. et al. previously studied this problem in detail using the example of the development of “no-reflow” after intervention on the coronary arteries. The authors describe the mechanisms involved in the development of the ischemia/reperfusion phenomenon, citing the following: disturbances in the autoregulation system, mechanical compression due to cell swelling with the development of intracellular and interstitial edema; in turn, tissue swelling can lead to compression of the ICR, disruption of the blood coagulation system, and microembolization of the ICB; additional pathogenetic factors (arterial hypertension, diabetes mellitus, etc.). Although these factors have been described for cardiac muscle, it is advisable to relate them to muscle tissue of the limbs. It is obvious that the “no-reflow” phenomenon is multifocal in nature and has many mechanisms, but the main role is played by microembolization and damage to the MCR during reconstructive interventions.
During the revascularization procedure, particles of atheromatous and thrombotic masses rush into more distal vessels, sometimes completely blocking the access of blood to the capillary. In the case of injurious reperfusion, plaque instability is of great importance. As a rule, natural microembolization is small, but the active destruction of the atherosclerotic plaque (in most cases with ACS - loose, unstable, with violations of the integrity of the tire) and fragmentation of the parietal thrombus on its surface are of great clinical importance. It can be assumed that in this case endovascular interventions pose a much greater danger. For example, with balloon angioplasty, mechanical crushing of the atherosclerotic plaque occurs. Balloon dilatation pushes fragments of atherosclerotic plaque into the muscular layer of the vascular wall, which leads to an expansion of the internal diameter of the vessel. However, some of the atheromatous masses may not move into the deep layers of the wall, but fragment in the lumen of the vessel. These fragments of plaque, as well as thrombotic layers, serve as a source of microembolization of distal vessels, while open surgical interventions are performed by clamping the arteries, therefore, eliminating the risk of atheromatous particles entering the distal bed during surgery. When treating patients with OAANK with acute obstruction of the main arteries of the extremities, it is necessary to take into account the extremely important fact that restoration of vessel patency (reperfusion) is almost never immediately accompanied by restoration of blood flow to the initial level [13], and the outcome of successful reconstructive surgery on the great vessels is largely due to tissue tolerance to reperfusion damage. The situation is complicated in the presence of diabetes mellitus, in particular diabetic foot syndrome [14]. In clinical practice, we often encounter reperfusion edema [15] due to the fact that the microvasculature, under conditions of increasing lactate concentration, increases its permeability, and the filtration/absorption balance is disturbed with a shift towards the former; under these conditions, the volume of filtrate exceeds the buffer capacity of the lymphatic system, therefore, swelling of the limb occurs. Edema is a direct consequence of disruption of the endothelial barrier, which is an easily recognized capillary response to the development of the inflammatory process during ischemia/reperfusion. The hyperpermeable state does not usually result from significant endothelial cell damage or detachment of endothelial cells from the vessel wall. On the contrary, this form of response reflects more subtle changes in the ultrastructure of the endothelial monolayer, such as expansion of endothelial paracellular contacts, which arise as a result of uncoupling of contact proteins and/or reduction of the cytoskeleton. The development of endothelial hyperpermeability, which accompanies the inflammatory process, is attributed to a variety of mediators that bind to specific endothelial receptors [16] and open the path of fluid into the extracellular space. Edema can be total, involving both muscles and subcutaneous tissue, or subfascial, affecting only the muscles [17]. In turn, edematous tissues become more easily infected and heal less well, which indicates an unfavorable prognosis for the course of the disease.
The most fatal reperfusion syndrome occurs in patients with acute ischemia of the limb, where the tissue has not had time to adapt to the decrease in blood flow; During the period of acute ischemia, the collaterals are also insufficiently opened, which reduces the compensatory reserve of the arterial segments of the lower limb. The frequency of reperfusion complications is high during reconstruction of the aortoiliac segment, since the volume of these interventions is almost always large, and the operation is performed by clamping the aorta. The development of “reperfusion syndrome” in the structures of a previously ischemic brain in the post-occlusion period in the form of cerebral edema and hemorrhages has also been established [18]. Sudden occlusion of the internal carotid artery impairs autoregulation and reduces blood flow in the homolateral hemisphere. When the main artery is occluded within 10-15 minutes, physicochemical changes occur in the blood: microembolism, post-ischemic edema of the endothelium of small vessels, disorders of their regulation, which, when artery patency is restored, causes the phenomenon of “non-resumption of perfusion” in 50% of the volume of the medulla [19] . The presence of reperfusion syndrome directly depends on the time during which tissue cells were exposed to hypoxic conditions. Rapidly developing ischemia is more pathogenic for cells than slowly progressing ischemia, since the latter leaves a certain time for adaptation and compensation [20].
In the mechanism of hypoxic necrobiosis, especially at deep stages, a key role is played by an increase in the content of ionized intracellular calcium, the excess of which is toxic to the cell. The increase in intracellular calcium concentration is initially due to a lack of energy to operate the calcium-magnesium pump. As hypoxia deepens, calcium enters the cell through the calcium input channels of the outer membrane, as well as through a massive flow from mitochondria, cisterns of the smooth endoplasmic reticulum and through damaged cell membranes. This leads to a critical increase in its concentration. A prolonged excess of calcium in the cytoplasm leads to activation of calcium-dependent proteinases and progressive cytoplasmic proteolysis. When the cell is irreversibly damaged, significant amounts of calcium enter the mitochondria, which leads to inactivation of their enzymes, protein denaturation, and permanent loss of the ability to produce ATP even when oxygen supply is restored or reperfusion. To summarize, we can say that the basis of reperfusion complications is the excessive supply of electrolytes - calcium, sodium, as well as water, glucose, oxygen and other substrates to altered or necrotic tissues that have lost the ability to metabolize them in typical redox reactions during recanalization of blood vessels. , as well as in the reactions of glycolysis, lipolysis, proteolysis. As can be seen, the main events associated with reperfusion occur at the level of the microcirculation, manifested by endothelial edema, leukocyte-endothelial adhesion, albumin extravasation and impaired arteriolar relaxation [21], and the main sensitive organs to reperfusion damage are the kidneys, brain, heart and lungs. The most common (48%) cardiac arrhythmia, mediated through reperfusion damage, occurs, namely extrasystole. Another manifestation of reperfusion syndrome is kidney damage. Incoming renal failure (creatinine above 0.13 mmol/l) is observed in 30-50% of patients undergoing elective surgery; in 1-2% of cases acute renal failure develops, which requires dialysis therapy [22].
It is worth noting that at the moment there are no recommendations for the management of patients with reperfusion complications; it is not indicated which methods or drugs are most rational to use for these purposes and in what dosage. There is no clear, pathogenetically substantiated, instrumentally and laboratory proven effectiveness of the use of various groups of drugs in the prevention of perioperative complications [23]. The main laboratory and diagnostic methods in the study of reperfusion processes are based on assessing the state of macrohemodynamics and microhemodynamics [24,25], as well as on the study of biochemical parameters of regional blood flow. So, Pshenikov A.S. et al. studied the level of nitric oxide (NO), vascular endothelial growth factor (VEGF), proapoptotic proteins (Bcl-2), heat shock proteins (HSP70) in patients with operable critical ischemia, as indicators of the functional and morphological state of the vascular system. According to the authors' study, as the severity of ischemia worsens, a significant decrease in these markers is observed. To predict reperfusion complications from the respiratory system, it makes sense to use coagulogram data: with a shift towards hypercoagulation, one should expect symptoms of respiratory failure when blood flow is restored. The most informative indicator of the state of microcirculation at the time of reconstructive interventions is transcutaneous oxygen tension. It was proposed to use gas transport drugs to protect cells from reperfusion damage at the time of interventions on the aorto-iliac segment. So, Voroshilin V.V. suggested using perftoran, because intravenously administered PF improves gas exchange and metabolism at the tissue level, increases the oxygen transport function of the blood, restores central and peripheral hemodynamics, improves the rheological properties of blood and microcirculation, causes disaggregation of platelets and erythrocytes, reducing the concentration of fibrinogen and factor XIII. In addition, it eliminates the effects of intravascular coagulation and recanalization of the vascular bed; being a membrane stabilizer, a mild osmodiuretic, a blocker of slow calcium channels, dissolving in membranes, it increases cell resistance to ischemic and reperfusion injury. One of the effects of perfluorocarbons that improve microcirculation is the formation of nitric oxide, which makes it possible to relieve vasospasm of the microcirculation [26,27]. However, the high cost of the drug (from 10,400.00 to 11,050.00 rubles per 200 ml in Moscow) does not allow its widespread use in all angiosurgery departments of the country, despite the fact that it is supposed to be used in a dosage of 5 ml/kg.
conclusions
Thus, the problem of reperfusion damage to organs is not solved. The most significant is the prevention of damaging tissue reoxygenation during the period of acute ischemia, where rapidly increasing ischemia can become a threat to the limb, and thromboembolectomy can provoke complications from the respiratory system, kidneys, heart, as well as the ongoing progression of limb ischemia.
The most common and relatively harmless manifestation of postoperative reperfusion is edema as a local disorder. The most unfavorable is the development of multiple organ failure with increasing severity of limb ischemia. Based on the foregoing, a solution to this problem is to protect sensitive organs to damaging reoxygenation, including the microvasculature, as well as to increase the gas transport function and improve the rheological properties of blood. It is necessary to provide for the development of this type of complications when performing operations for limb ischemia, both acute and chronic.
Literature
- Gavrilenko A.V., Shabaltas E.D. The state of microcirculation in reperfusion syndrome after reconstructive operations on the vessels of the lower extremities. Surgery, 2003, No. 2, pp. 62-65.
- Holmberg A, Sandhagen B, Bergqvist D. Hemorheologic variables in critical limb ischemia before and after infrainguinal reconstraction. J Vase Surg 2000, V.31, P.691-695.
- Molecular mechanisms of damage. Methodological developments for independent work of students of medical universities. Ed. Prof. G.V. Poryadina. M.: RGMU, 2009.
- Churilov L.P. General pathophysiology with the basics of immunopathology. Textbook for medical university students. 5th edition. SPb.: ELBI-SPb, 2015, 256 p.
- Harkin DW, Barros D'Sa AA, McCallion K, et al. Bactericidal/permeability-increasing protein attenuates systemic inflammation and acute lung injury in porcine lower limb ischemia-reperfusion injury. Ann Surg 2001, Vol. 234, No. 2, P.233-244.
- Neimark M.I., Merkulov I.V., Flat M.K. Protection of renal function during surgical treatment of chronic infrarenal aortic aneurysms. Anesthesiology and Resuscitation, 2005, No. 4, pp. 18-22.
- Hernandez LA, Grisham MB, Twohig B, et al. Role of neutrophils in ischemia/reperfusion-induced microvascular injury. Am J Physiol 1987, 253: H699-H703.
- Lundgren O, Haglund U. Intestinal ischemia and shock factors. Fed Proc, 1978; 37: 2729-33.
- Bilenko M.V. Ischemic and reperfusion injuries of organs (molecular mechanisms, ways of prevention and treatment). M.: Medicine, 1989. 368 p.
- Kryzhanovskaya S.A., Matyushin G.V., Protopopov A.V. The “no-reflow” phenomenon: frequency, causes, clinical manifestations.
- Manukhina E.B., Terekhina O.L., Belkina L.M. and others. Vasoprotective effect of adaptation in ischemic and reperfusion injury of the heart. Pathological physiology and experiment. Therapy, 2013, No. 4, pp. 26-31.
- Karpov R.S., Dudko V.A. Atherosclerosis: pathogenesis, clinical picture, functional diagnostics, treatment. Tomsk: STT, 1998, 672 p.
- Kalinin R.E. and others. Reperfusion tissue damage in surgery of the arteries of the lower extremities. News of surgery, 2015, No. 3, pp. 348-352.
- Abalmasov K.G., Buziashvili Yu.I., Morozov K.M. Quality of life of patients with chronic ischemia of the lower extremities. Angiology and vascular surgery, 2004, Volume 10, No. 2, P.8-13.
- Suchkov I.A. and others. Prevention of restenosis in reconstructive surgery of the great arteries. Science of the Young/Eruditio Juvenium, 2013, No. 2, pp. 12-19.
- National recommendations for the management of patients with diseases of the arteries of the lower extremities. M., 2013, 74 p.
- Sologub T.V. and others. Free radical processes and inflammation (pathogenetic, clinical and therapeutic aspects). Training manual for doctors. M.: Academy of Natural Sciences, 2008.
- Suzuki H, Schmid-Schonbein GW, Suematsu M. Impared leucocyte-endothelial cell interaction in spontaneously hypertensive rats. Hypertension, 1994, V.24, P.719-727.
- Merkulov I.V., Neimark M.I. Anesthesia and intensive care in surgery of the aorta and its branches: monograph. Petrozavodsk: IntelTek, 2005, 272 p.
- Kuznetsov M.R., Koshkin V.M., Komov K.V. Modern aspects of diagnosis, prevention and treatment of reperfusion syndrome. Angiology and vascular surgery, 2006, T.12, No. 1, P.133-143.
- Savelyev V.S., Koshkin V.M. Critical ischemia of the lower extremities. M.: Medicine, 1997, 160 p.
- Dick F, Li J, Giraud MN, et al. Basic Control of Reperfusion Effectively Protects Against Reperfusion Injury in a Realistic Rodent Model of Acute Limb Ischemia. Circulation, 2008, Vol.118, No.19, P.1920-1928.
- Shcherbak N.S., Galagudza M.M., Yukina G.Yu. et al. Morphofunctional changes in pyramidal neurons of various fields of the hippocampus during ischemic postconditioning. Morphology, 2013, No. 3, pp. 7-13.
- Barsukov A.E., Makhnov HA Endothelial dysfunction: principles of diagnosis and clinical significance in obliterating atherosclerosis of peripheral arteries. Bulletin of Surgery, 2005, T.164, No. 1, P.102-104.
- Polyantsev A.A., Mozgovoy P.V., Frolov D.V. and others. Prediction of complications after reconstructive operations on the aortoiliac segment. Surgery, 2004, No. 4, pp. 9-12.
- Zimon I.N., Mavlyanova N.A. Respiratory disorders in patients with acute arterial obstruction and ischemic syndrome of the lower extremities. Surgery, 1997, No. 7, pp. 16-18.
- Sergienko V.I., Petrosyan E.A., Onopriev V.I. and others. Morphological changes in the lungs during modeling and treatment of ischemic and reperfusion injuries of the limb. General resuscitation, 2006, T.II, No. 5-6, P.129-132.
Comments on the article
Article rating:
0/5
(no ratings)
No comments