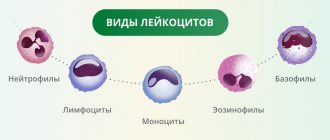
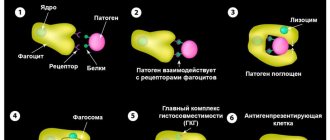
Monocytes are one of the types of white blood cells. Their functionality is of great importance for ensuring and maintaining the health of the human body.
They perform such important functions as antitumor, antiviral and others. However, often after taking blood tests you can hear from the doctor that the level of monocytes is increased. Unfortunately, few people know what this statement means and how to correct the situation.
And, most importantly, does such a phenomenon pose any danger in the body.
What are monocytes, the mechanism of their formation
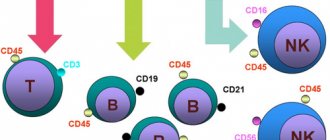
Monocytes are white blood cells with a diameter of 18 to 20 microns, belonging to agranulocytic leukocytes. These are the largest cells in the peripheral bloodstream. Microscopically, a monocyte is defined as an oval cell with one polymorphic nucleus, shaped like a bean. The core is in an eccentric position. The pronounced coloring of the monocyte nucleus distinguishes it from lymphocytes. When assessing a blood test, this sign allows you to correctly assess the blood picture. Healthy indicators of monocytes are 3-11% of the total volume of white blood cells.
A significant number of these cells are determined in:
- spleen;
- liver;
- lymph nodes;
- bone marrow.
The formation, development and growth of monocytes proceeds to the bone marrow under the influence of active substances:
- Glucocorticosteroids that can slow down the synthesis of monocytes.
- Cell growth factors GM-CSF and M-CSF, which stimulate the development of monocytes.
Formed mature monocytes function in the bloodstream for 2-3 days. Some of them die during this period due to physiological apoptosis, which is provided for by normal natural processes in the body. The remaining cells transform into macrophages and spread throughout internal organs and tissues. The life cycle of these advanced cells is 1 to 2 months.
Special qualities of monocytes
The bulk of monocytes are synthesized in the bone marrow. The first to emerge from a multipotent stem cell is a monoblast. Subsequently, it goes through the promyelomonocyte phase, and then the promonocyte phase. Promonocytes in the last stage of maturation have a pale, loose nucleus; traces of nucleoli are visible in the cytoplasm. Azurophilic granules are detected in mature monocytes and promonocytes. Both types of cells belong to the agranulocytic type. Granules of immature cells, histogenic particles and lymphocytes are the result of protein discolloidosis of the cytoplasm and are stained with azure using the Romanovsky-Giemsa method. In the connective tissue of internal organs and lymph nodes, a small part of monocytes is formed.
Biologically active substances and hydrolytic enzymes were found in the cytoplasm of mature monocytes:
- Lipase.
- Protease.
- Verdoperoxidase.
- Carbohydrase.
Lactoferrin and myeloperoxidase are found in minimal quantities.
The human body is able to activate the synthesis of monocytes in the bone marrow slightly. The division of phagocytic mononuclear cells outside the bone marrow occurs extremely slowly and is limited. The mechanism for replacing dead specific cells is possible only with the help of monocytes spreading through the bloodstream. In peripheral blood, monocytes function for about 72 hours, after which they penetrate into the tissues of nearby organs. Here the process of their transformation into mature histocytes, highly differentiated macrophages - Kupffer cells of the liver, alveolar cells of the lungs and other organs is completed.
Non-professional phagocytes:
This group of cells includes several other types of phagocytes, which do not have a directed effect against specific “enemies”, since they do not know how to present antigens. They have phagocytic activity, but it is less pronounced than the other cells described above. Examples include skin cells, some elements of connective tissue, etc. There are animals whose retina and even eggs are capable of phagocytosis!
So, in the tissues and blood of the body there is a large
number of phagocyte cells. They interact with each other in a complex manner, and in some cases their correct interaction may be disrupted, which will lead to health problems.
Knowing the fragility of this system, it is important to prevent imbalances in it. To do this, you need to monitor your general condition, lead a correct lifestyle, be sure to immediately and completely treat all emerging diseases, and also take special means to support the immune system.
Transfer Factor drug
in this case, it is optimal: it has a unique immunomodulatory effect. The information molecules contained in it carry a huge baggage of immunological memory accumulated by many generations of living beings. The value of this information is increased by the fact that it is presented in a “language” familiar to our immune system, since it was obtained during the activity of the same phagocyte cells.
Information molecules transmit the available data to immune cells, which distribute it among themselves. This becomes an impetus for the normalization of organ function and the body’s natural self-healing, which is the key to strengthening and maintaining overall health.
Differences between macrophages and monocytes
Until the 70s of the 20th century, scientists were convinced that all monocytes eventually transform into macrophages. Any additional sources of the appearance of “cleaners” have not been identified. And only in 2008, after a series of studies, it was established that macrophages have different origins. Some of them are transformed from monocytes, the rest develop from precursor cells of various types during intrauterine development of the fetus.
The transformation of one cell into another goes through certain stages. After monocytes enter the internal tissues from the blood, their active growth begins. At the same time, the development of mitochondria and lysosomes occurs. Thanks to these internal rearrangements, monocyte macrophages acquire specific properties.
Dendritic cells:
Perhaps some readers are seeing such a name for the first time and previously did not even suspect that such cells even exist. Nevertheless, they exist, and are located in large quantities in human tissues. As a rule, they are located close to the external environment and organ cavities: under the gastric mucosa, in the alveolar tissue of the lungs, in the nasal mucosa, etc. They were called dendritic because their numerous long processes give them a branching structure and make them look like trees (dendron - tree).
In these tissues, cells mature, after which they are sent to areas of accumulation of lymphoid tissue, interact with lymphocytes and macrophages and increase their activity.
Biological role in the body
The biological role of monocytes is very diverse. The largest phagocytes are necessary for many functions:
- Phagocytosis.
During this process, monocytes and macrophages identify proteins, bacteria, and viruses that are dangerous to the body, capture them and absorb them (phagocytose).
- Participation in the development of specific immunity.
Monocytes produce special cytotoxins, interferon, to counteract pathogenic viruses, bacteria and fungi.
- Assistance in the formation of allergic reactions.
Thanks to monocytes, specific elements of the compliment system are produced. Due to these processes, foreign proteins - allergens - are identified.
- Protection against tumors.
It is carried out through the production of tumor necrosis factor.
- Regulation of hematopoiesis and blood clotting processes
- carried out through the synthesis of specific elements.
Monocytes have special characteristics that distinguish them from neutrophils and other phagocytes:
- After absorbing a foreign element, monocytes do not die, but continue to function actively. In rare cases, the pathogen can defeat them.
- The life cycle of monocytes is longer than that of neutrophils.
- Monocytes have a pronounced antiviral effect, neutrophils have an antibacterial effect.
- When many monocytes are activated against a foreign agent, pus does not form.
- Chronic inflammatory process is a favorite place for concentration of macrophages and monocytes
The norm of monocytes in the blood
The rate of monocytes in peripheral blood ranges from 3 to 11%, which in absolute values is 0.04-0.08*109/liter. According to generally accepted medical decoding, monocytes are designated MON. Depending on the age of the person, their norm changes. Normal values are not determined by the gender of the patient.
| Person's age | Norm of MON parameters |
| from 1 to 15 days | 5%-15% |
| from 15 days to 1 year | 4%-10% |
| from 1 to 2 years | 3%-10% |
| from 2 to 15 years | 3%-9% |
| over 15 years old | 3%-11% |
A physiological increase in monocytes can be observed in women during pregnancy, but they do not go beyond normal limits.
The absolute volume of monocytes in one liter of blood is of great importance. These indicators differ for children and adults:
- Children under 12 years old - from 0.05 to 1.1 * 109 / liter.
- Over 12 years old - from 0.04 to 0.08*109/liter.
Reasons for the increase in monocytes in the blood
A detected increase in the level of monocytes above the established age norm is defined as monocytosis.
There are two types of this condition:
- Absolute monocytosis
characterized by an isolated increase in monocytes. Their level increases in adults by more than 0.8*109/l, for children under 12 years of age - more than 1.1*109/l. This symptom progresses against the background of certain pathologies in which the production of special phagocytes is activated.
- Relative monocytosis
characterized by a normal absolute value of monocytes, but an increase in their percentage in the blood. This occurs with a combined drop in the concentration of other leukocytes.
The state of absolute monocytosis raises alarming concerns among doctors, as it indicates a pathological process. Relative monocytosis is a temporary phenomenon and goes away on its own. In any case, an increase in the concentration of monocytes indicates that the body is resisting foreign elements.
Physiological causes of monocytosis
An increase in the monocyte index is observed in the first two hours after eating. This is a normal process for all people. Doctors insist that a general blood test be taken in the morning on an empty stomach, precisely for this reason.
An increase in monocytes in women is recorded in special physiological conditions:
- Menstruation.
At the beginning of the cycle, active rejection of endometrial cells occurs. In response to this, blood monocytes and macrophages rush to the site to perform their main function - cleansing. The highest concentration of monocytes is recorded during the period of heaviest bleeding. Independent normalization of phagocytes occurs after the end of bleeding. To get a more accurate picture, you should not take blood for a general analysis during menstruation.
- Pregnancy.
In the first three months of pregnancy, an active transformation of a woman’s immunity begins. Due to all the changes, there is a temporary decrease in the level of monocytes. Gradually their concentration changes and reaches a maximum in the third trimester, especially before childbirth. It should be noted that the peak values do not exceed the limits of normal values for the corresponding age.
When a general blood test reveals an increase in monocytes above normal levels, the state of monocytosis is defined as pathological. In such cases, professional medical assistance is necessary.
Infectious diseases
An increase in phagocytes occurs during infectious diseases. For example, in acute respiratory viral infections, the values of monocytes in a general blood test exceed the age limits several times. During the action of bacterial pathogens, there is an increase in neutrophils, and during viral pathogens, monocytes are used. The activity of phagocytes persists throughout the illness and for 2-4 weeks after recovery.
The persistence of monocytosis for 6-8 weeks or longer indicates a chronic course of the infectious disease. A slight excess of the upper norm is observed during colds - up to 0.09-1.5*109/l. A pronounced increase in monocytes up to 30-50*109/l may indicate the development of oncohematological diseases.
In pediatrics, monocytosis most often becomes a companion to infectious diseases.
Infectious mononucleosis
A common disease caused by the herpes-like Epstein-Barr virus. For adults, the infection is not dangerous due to the developed immune system.
The clinical picture progresses sharply and is characterized by the following manifestations:
- Fever up to 38-40 °C is characterized by a long course with periods of improvement and exacerbation. Features of temperature are the property that distinguishes the disease from ARVI.
- Rhinitis, sore throat.
- Hypertrophy of the lymph nodes in the occipital and submandibular region without pain.
- Skin rashes.
- Expansion of the boundaries of the spleen and liver.
- In the CBC there is an increased level of monocytes and lymphocytes.
To clarify the diagnosis, it is possible to perform an analysis for the presence of specific antibodies. During treatment, targeted antiviral drugs are not prescribed. Symptomatic therapy is carried out.
Childhood infections
Most childhood infections are transmitted by humans once and do not occur after adulthood. They are characterized by increased levels of monocytes and lymphocytes. This phenomenon is observed with a prolonged course of rubella, measles, whooping cough, and mumps.
Monocytosis in adults may be a reaction to the progression of more severe infectious diseases:
- Tuberculosis.
- Cytomegalovirus infection.
- Brucellosis.
- Syphilis.
- Typhoid fever.
- Sarcoidosis.
Helminthiasis
When parasites enter the body, they receive a quick response from the circulatory system. Monocytes are the first to react and begin to fight opisthorchiasis, roundworms, pinworms, bovine or pork tapeworms. Parasites familiar to our latitudes and exotic ones primarily affect the intestines.
Infestations manifest themselves in the form of specific symptoms:
- Sharp pain in various parts of the abdomen.
- Dyspeptic breakdown like diarrhea.
- Loss of body weight while maintaining or increasing appetite.
- Skin allergy in the form of urticaria.
In the blood, along with monocytosis, an increase in the level of granulocytic leukocytes - eosinophils, which regulate the formation of an allergic response, is recorded. To determine the type of parasite, stool is taken from the patient, bacterial cultures and immunological tests are performed. Therapy is specific and consists of taking antiparasitic drugs.
Chronic infectious and inflammatory diseases
Prolonged infection with unexpressed symptoms weakens the body, causing an increase in monocytes in the blood and the concentration of macrophages in the internal organs. The development of the clinical picture is influenced by the localization of the infectious process and the type of pathogen.
Monocytosis can be a symptom of an infection of the throat, lungs, bones, bacterial effects on the myocardium, kidneys, pelvic organs, and gall bladder. The course of chronic diseases is accompanied by periodic or constant pain in the affected area. An increase in temperature does not always appear. The success of adequate treatment depends on prompt identification of the cause. Therapeutic measures help relieve symptoms and restore the physiological level of monocytes in the blood.
Autoimmune disorders
Under the influence of certain factors, the immune system can become distorted. These disorders cause immune cells to identify their own elements as foreign and attack them. In autoimmune diseases, macrophages and monocytes rush to joints, kidneys, skin, and cardiac tissues, which appear as suspicious lesions.
The most common autoimmune disorders include:
- Diffuse toxic goiter, the main symptom of which is the active synthesis of thyroid hormones.
- Rheumatoid arthritis causes joint damage.
- Systemic lupus erythematosus is a serious disease that affects the tissues of internal organs, joints, and skin.
- Systemic scleroderma primarily affects the skin and then spreads to internal tissues.
- Diabetes mellitus (insulin-dependent type) causes a disorder in glucose metabolism and subsequent metabolic disorders throughout the body.
The detection of monocytosis in a blood test is not a clear symptom of an autoimmune disease. If the reasons for the shift in blood composition are not clear, additional diagnostic measures should be performed.
Oncohematological diseases
Malignant changes in the hematopoietic system are always accompanied by pronounced monocytosis. For their treatment, serious protocols are used, which are not always effective. After fixing an excessive level of monocytes, the patient should exclude autoimmune and infectious diseases. If the reasons for the changes cannot be established, there is a need to consult an oncohematologist.
Monocytosis can be a manifestation of serious diseases of the hematopoietic system:
- Acute leukemia of monocytic or myelomonocytic type.
With this pathology, monocyte precursors are determined in blood and bone marrow tests. The majority of patients are children under two years of age. Symptoms: anemia, bleeding, frequent infectious diseases, pain in bones and joints. The disease is characterized by an unfavorable course.
- Multiple
myeloma.
The main patients are people over 60 years of age. Symptoms: bone pain, frequent fractures, bleeding, decreased protective properties of the body.
Oncohematological disorders lead to a fairly high content of monocytes in the blood - more than 30-50*109/l. Indicators of this level give reason to believe that monocytosis is caused not by infectious factors, but by a malignant process. In the case of chronic inflammation, monocytes increase slightly, and in myeloma and leukemia, the number of agarnulocytes suddenly reaches high values.
Other cancers
Hodgkin's disease is one of the pathologies that should be excluded in case of monocytosis. Lymphogranulomatosis can manifest itself in the form of focal signs of internal organs, high fever, and hypertrophy of the lymph nodes. Symptoms of spinal cord damage may appear. In order to make a correct diagnosis, a biopsy of the lymph nodes is performed for histological analysis of the contents.
Monocytosis can be detected in the blood in various types of tumors. Detailed diagnostics using hardware research methods helps determine the nature and localization of the malignant process.
Intoxication with chemical reagents
Monocytosis can occur under specific conditions:
- Tetrachloroethane poisoning.
A toxic substance can enter the body through the upper respiratory tract, orally or by contact. Signs of intoxication: headache, yellowness of the skin, irritation of the mucous membranes. In severe cases, it causes liver failure and coma.
- Phosphorus poisoning.
Intoxication occurs when a toxic substance is ingested orally or by air. Symptoms: severe abdominal pain, diarrhea, nausea. The lack of adequate therapy leads to irreversible changes in the kidneys, liver, and central nervous system.
In case of poisoning, not only monocytosis is recorded, but also other pathological abnormalities in laboratory tests.
Read more: Monocytes are elevated: what does this mean? What to do?
Symptoms
How will a person feel, how will he feel? What should motivate a person to go and take a blood test? What symptoms might he have that could lead to an increase in monocytes in the blood?
- This could be causeless weight loss.
- Decreased or complete absence of appetite.
- Increased fatigue. Unreasonable weakness. It seemed like he had gotten enough sleep, he didn’t seem to be overtired, he seemed to be resting, but in the morning he got up and had no strength. This groundless weakness requires mandatory monitoring of blood tests.
- Anxiety, panic attacks, depression, or psycho-emotional agitation. This should also encourage a person to go and do a detailed blood test.
- Irritability, apathy, drowsiness - any disturbance in the psycho-emotional state can cause changes in the peripheral blood.
- Sudden refusal of meat food, even to the point of disgust for meat food. As a rule, monocytosis will be present in a detailed blood test.
- Gastrointestinal disorders: bloating, rapid motility, bowel dysfunction, both constipation and diarrhea, increased secretion of mucus in feces.
- A dry, prolonged cough, sometimes streaked with blood, with scanty sputum.
- Joint and muscle pain in the back and legs. This also requires decoding and examination.
- Specific rashes on the skin and mucous membranes can also be a condition in which there will be monocytosis or an increase in the concentration of monocytes in the blood.
- Presence of rashes on the genitals.
It must be said that all of the above reasons can be disturbing, both individually and collectively, together. Several symptoms unite into entire syndromes. I want to emphasize that an increase in the number of monocytes in the blood in itself is not a diagnosis. This is a state of peripheral blood cells of the immune system, which indicates that not everything is in order in the “state”, and this requires mandatory examination, requires careful attention and further decoding.
Reasons for the decrease in monocytes in the blood
A decrease in monocyte levels in the blood below the appropriate age norm is called monocytopenia.
The cause of such changes in the composition of the blood may be certain conditions:
- Aplastic anemia.
- Purulent infections of bacterial origin.
- Late stages of oncohematological pathologies.
- Long-term use of medications.
Purulent bacterial infections
With purulent diseases caused by streptococcus or staphylococcus, the body becomes infected and an inflammatory process develops.
Among the infections that cause such changes, the following should be highlighted:
- Skin diseases - furunculosis, phlegmon, carbuncle.
- Osteomyelitis is a purulent bone lesion.
- Bacterial pneumonia.
- Sepsis is a severe blood infection characterized by a worsening of the patient’s general condition.
Aplastic anemia
A disease of the hematopoietic system that occurs as a result of inhibition or arrest of the growth and maturation of monocytes, red blood cells, leukocytes and platelets in the bone marrow. Symptoms: general weakness, decreased performance, dizziness, increased heart rate, constant pallor of the skin, bleeding, frequent infectious diseases, weakening of the body's protective properties.
In the absence of specific therapy, aplastic anemia leads to the death of the patient. This serious disease is treated by using cytostatics, hormonal drugs, and in severe cases a bone marrow transplant is required.
Oncohematological pathologies
Severe, long-term course of leukemia causes suppression of hematopoietic processes. Against this background, pancytopenia develops - a deficiency of all blood cells, including monocytes. The patient may experience severe infections due to immune disorders. Bleeding of unknown etiology often develops. Only timely bone marrow transplantation can stop the deterioration of the situation and promote the patient’s recovery.
Treatment with certain drugs
Long-term use of cytostatics and corticosteroids can lead to a deterioration in the hematopoietic activity of the bone marrow. This manifests itself in negative changes in blood composition. If a reduced content of certain blood cells is detected, the drug is discontinued and appropriate treatment is carried out. Timely measures help restore the normal state of the bone marrow.
Read more: Monocytes are low: what does this mean? How to increase?
According to the “M1/M2” paradigm, there are two subtypes of activated macrophages—classically activated (M1) and alternatively activated (M2), which express various receptors, cytokines, chemokines, growth factors, and effector molecules. However, recent data indicate that, in response to changes in microenvironmental signals, macrophages may exhibit unique properties that do not allow them to be classified into any of these subtypes.
Macrophages play a major role in the body's response to implanted material - catheters, stents, endoprostheses, dental implants. Macrophages phagocytize wear particles from the surface of joint prostheses, initiate inflammation in the prosthetic area and osteolysis, and control the formation of a fibrous capsule around foreign bodies. A brief overview of the factors causing migration, adhesion and activation of macrophages, analysis of their functional characteristics on various surfaces, including biodegradable and non-degradable materials in vivo and in vitro, is presented.
Introduction
Modern medicine is currently impossible to imagine without the use of implantable products installed in the body for various periods of time in order to restore the anatomy and function of organs and tissues lost or affected by a pathological process. The biocompatibility of synthetic materials or tissue-engineered constructs is a major issue affecting the results of such implantations. The reaction to prosthetic material develops in the following sequence: tissue alteration, infiltration by cells of acute, then chronic inflammation with the formation of granulation tissue and fibrous capsule. The severity of these reactions determines the biocompatibility of the implanted device. Macrophages play a major role in the body’s reaction to the installed material - catheters, stents, endoprostheses, dental implants, etc.
Morphology of macrophages
Macrophages are a heterogeneous cell population. The macrophage has an irregular, stellate, multi-processed shape, folds and microvilli on the cell surface, an abundance of endocytic microvesicles, primary and secondary lysosomes. The round or ellipsoid nucleus is located centrally, heterochromatin is localized under the nuclear envelope. The structural features of a cell largely depend on its organ and tissue affiliation, as well as on its functional status. Thus, Kupffer cells are characterized by a glycocalyx, alveolar macrophages contain lamellar (surfactant) bodies, a well-developed Golgi complex, a rough endoplasmic reticulum and many mitochondria, while in microglial cells there are few mitochondria. In the cytoplasm of peritoneal and alveolar macrophages there is a large number of lipid bodies containing substrates and enzymes for the generation of prostaglandins [1]. Adhering and moving macrophages form short-lived, actin-containing structures - podosomes - in the form of a dense central part with microfilaments radiating from them. Podosomes can fuse to form higher-order structures—rosettes—that effectively destroy proteins in the underlying extracellular matrix[2].
Functions of macrophages
Macrophages phagocytose foreign material and cellular tissue detritus, stimulate and regulate the immune response, induce an inflammatory response, and participate in reparative processes and the exchange of extracellular matrix components. The variety of functions performed explains the expression by these cells of a large number of receptors associated with the plasma membrane, intracellular and secreted. Innate immune receptors PRR (pattern-recognition receptors) are activated by a wide range of ligands (with the exception of CD163), providing recognition of highly conserved structures of most microorganisms, the so-called PAMPs (pathogen-associated molecular patterns, pathogen-associated patterns) and similar with them endogenous molecular structures DAMP (damage-associated molecular patterns), formed as a result of damage and cell death, modification and denaturation of protein structures of the extracellular matrix. Most of them mediate endocytosis and elimination of potentially dangerous endogenous and exogenous agents, but at the same time, many of them perform signaling functions, regulating the synthesis of proinflammatory mediators, promoting adhesion and migration of macrophages (Table) [3–7].
The plasma membrane of monocytes/macrophages also expresses specialized receptors that bind one or more structurally similar ligands: the Fc fragment of immunoglobulin G, growth factors, corticosteroids, chemokines and cytokines, anaphylotoxins and costimulatory molecules. The functions of many of these receptors are mediated not only by the binding of ligands, but also by interaction with other receptors (C5aR-TLR, MARCO-TLR, FcγR-TLR), which ensures fine regulation of the synthesis of pro- and anti-inflammatory mediators [2, 6, 8, 9]. A feature of the macrophage receptor system is the presence of trap receptors for proinflammatory cytokines and chemokines (Il-1R2 on M2a macrophages; CCR2 and CCR5 on M2c macrophages), the activation of which blocks the intracellular transmission of the corresponding proinflammatory signal. The expression of cellular receptors is species-, organ- and tissue-specific and depends on the functional status of macrophages. Macrophage cellular receptors studied in detail are shown in the table.
Migration of monocytes/macrophages
Tissue macrophages are derived primarily from blood monocytes, which migrate into tissues and differentiate into different populations. Macrophage migration is directed by chemokines: CCL2 CCL3, CCL4, CCL5, CCL7, CCL8, CCL13, CCL15, CCL19, CXCL10, CXCL12; growth factors VEGF, PDGF, TGF-b; fragments of the complement system; histamine; granule proteins of polymorphonuclear leukocytes (PMNL); phospholipids and their derivatives.
At the initial stages of the inflammatory response, PMNs organize and modify a network of chemokines by secreting CCL3, CCL4 and CCL19 and releasing azurosidine, LL37 protein, cathepsin G, defensins (HNP 1-3) and proteinase 3 preformed into granules, which ensure the adhesion of monocytes to the endothelium, thereby thereby exhibiting the properties of chemoattractants. In addition, PMN granule proteins induce the secretion of chemokines by other cells: azurosidine stimulates the production of CCL3 by macrophages, and proteinase-3 and HNP-1 induce the synthesis of CCL2 by the endothelium. PMN proteinases are capable of activating many protein chemokines and their receptors. Thus, proteolysis of CCL15 by cathepsin G greatly enhances its attractive properties. Apoptotic neutrophils attract monocytes through signals presumably mediated by lysophosphatidylcholine[10].
Any tissue damage leads to the accumulation of macrophages. In the area of vascular injury, the blood clot and platelets release TGF-β, PDGF, CXCL4, leukotriene B4 and IL-1, which have pronounced chemoattractive properties against monocytes/macrophages [11–13]. Damaged tissues are a source of so-called alarmins, which include components of the destroyed extracellular matrix, heat shock proteins, amphoterin, ATP, uric acid, IL-1a, IL-33, mitochondrial DNA of cellular debris, etc. They stimulate the remaining viable cells of damaged tissues and the endothelium of blood vessels to the synthesis of chemokines, some of them are direct factors of chemotaxis [13, 14]. Infection of tissues leads to the appearance of so-called pathogen-associated molecules: lipopolysaccharides, cell wall carbohydrates and bacterial nucleic acids. Their binding by membrane and intracellular receptors of macrophages triggers the process of expression of chemokine genes, which provide additional recruitment of phagocytes [11, 15].
Macrophage activation
Macrophages are activated by a variety of signaling molecules, causing their differentiation into various functional types (Fig. 1). Classically activated macrophages (M1 phenotype) are stimulated by IFNg, as well as IFNg together with LPS and TNF. Their main functions are the destruction of pathogenic microorganisms and the induction of an inflammatory response. Polarization in the M1 direction is accompanied by the secretion of proinflammatory mediators. They express receptors for IL-1 – IL-1R1, TLRs and co-stimulatory molecules, the activation of which ensures amplification of the inflammatory response. Along with pro-inflammatory cytokines, macrophages also secrete the anti-inflammatory cytokine IL-10, with a characteristically high IL-12/IL-10 ratio [11, 13, 16–18]. The bactericidal properties of M1 macrophages are determined by the production of free radicals of nitrogen and oxygen generated by iNOS and the NADPH oxidase complex [19, 20]. Being effector cells in the body's response to bacterial infection, they, at the same time, suppress the adaptive immune response by inhibiting the proliferation of stimulated T cells. IL-12 secreted by M1 macrophages plays a key role in Th1 polarization, and IL-1b and IL-23 direct the immune response along the Th17 pathway [11, 18]. Recent studies have shown that M1 macrophages, in addition to pro-inflammatory properties, exhibit reparative properties: they secrete VEGF, which stimulates angiogenesis and the formation of granulation tissue [21].
Alternative activation of macrophages (M2 phenotype) is observed when they are stimulated by interleukins, glucocorticoids, immune complexes, TLR agonists, etc. They migrate to zones of helminth invasion, accumulate in fibrosis loci, in healing skin wounds and neoplastic formations. M2 macrophages are capable of active proliferation in situ. They exhibit a greater ability for phagocytosis compared to M1 macrophages and express a greater number of associated receptors: CD36 – scavenger receptor of apoptotic cells; CD206 – mannose receptor; CD301 – receptor for galactose and N-acetylglucosamine residues; CD163 is a receptor for the hemoglobin-haptoglobin complex. Macrophages of this type are characterized by a low IL-12/IL-10 ratio[11, 22–25].
Alternatively activated macrophages are divided into subtypes: M2a, M2b and M2c. An example of the M2a phenotype of macrophages are cells that accumulate around helminth and protozoan larvae, the allergens of which induce an immune Th2 response, accompanied by the production of IL-4 and IL-13[23]. They do not secrete significant amounts of proinflammatory cytokines and synthesize a special spectrum of chemokines and membrane receptors [11, 17, 22]. It is believed that they are characterized by the synthesis of IL-10[13], however, in vitro, macrophages do not always produce this cytokine and can exhibit high transcriptional activity of the IL-12 and IL-6 genes[17, 26]. An important characteristic of this population is the synthesis of IL-1 receptor antagonist (IL-1ra), which, by binding to IL-1, blocks its proinflammatory effects[27].
M2a macrophages suppress the inflammatory response by blocking the formation of the M1 population through the cytokines of the Th2 lymphocytes recruited by them, or due to the chemokine CCL17 produced, which, together with IL-10, inhibits the differentiation of macrophages in the M1 direction [11]. M2a phenotype cells are considered typical reparative macrophages. The chemokine CCL2 synthesized by them is a chemoattractant of myofibroblast precursors – fibrocytes[28, 29], they secrete factors that ensure connective tissue remodeling[20, 22, 24, 30–32].
Polarization in the M2b direction is accomplished by stimulation of the Fcg receptor together with TLR agonists and ligands for the IL-1 receptor. Functionally, they are close to M1 macrophages, producing pro-inflammatory mediators and nitrogen monoxide (NO), but at the same time they are characterized by a high level of IL-10 synthesis and reduced IL-12 production [16, 17]. M2b macrophages increase antibody production. The chemokine CCL1 synthesized by them promotes the polarization of lymphocytes in the Th2 direction [11, 13]. M2c macrophages have suppressive properties - they inhibit the activation and proliferation of CD4+ lymphocytes caused by antigenic stimulation and promote the elimination of activated T cells [33]. In vitro, the M2c subtype is obtained by stimulating mononuclear phagocytes with glucocorticoids, IL-10, TGF-β, prostaglandin E2, etc. They do not have bactericidal activity, produce a small amount of cytokines, secrete growth factors and some chemokines [7, 13, 17, 21, 31 ]. M2c macrophages express receptors for phagocytosis and many proinflammatory chemokines, which presumably do not serve to excite the corresponding signals, but are traps for proinflammatory mediators, blocking their functions [11].
The nature of macrophage activation is not strictly determined and stable. The possibility of transformation of the M1 phenotype into M2 has been shown with a change in the spectrum of stimulating cytokines and due to efferocytosis. After engulfing apoptotic cells, macrophages sharply reduce the synthesis and secretion of inflammatory mediators CCL2, CCL3, CXCL1, CXCL 2, TNF-a, MG-CSF, IL-1b, IL-8 and greatly increase the production of TGF-b [20, 25, 27] . The reverse transformation of the M2 phenotype to M1 is expected during the development of obesity.
Many authors question the existence in the body of two clearly distinguishable populations of macrophages M1 and M2. A combination of signs of classical and alternative activation is characteristic of macrophages of human skin wounds. Thus, along with the cytokines TNF-a and IL-12 typical for M1 macrophages, they demonstrate the synthesis of M2 macrophage markers: IL-10, CD206, CD163, CD36 and receptors for IL-4 [34]. A type of macrophages different from M1/M2 with pronounced fibrinolytic activity was found in the liver of mice in a model of reversible fibrosis and in human liver tissue with cirrhosis. They express the genes of arginase 1, mannose receptors and IGF, they secrete MMP-9, MMP-12, exhibit a pronounced ability to proliferation and phagocytosis, but do not synthesize IL-10, IL-1ra, TGF-b[35]. A special population of macrophages is formed in the mouse spleen during infection with mycobacteria. They inhibit the proliferation of T lymphocytes and their secretion of both Th1 and Th2 cytokines, stimulating polarization in Th17. direction. Suppressive macrophages have a unique phenotype - they express genes active in M1 macrophages - IL-12, IL-1b, IL-6, TNF-a, iNOS and at the same time genes CD163, IL-10, mannose receptors and other markers of M2 macrophages [19].
These studies clearly show that the macrophage populations formed in natural conditions differ significantly from the M1 and M2 populations obtained in vitro. Perceiving a variety of activating signals, the macrophage responds “on demand”, secreting mediators adequately to changes in the environment, therefore, in each specific case, its own phenotype is formed, sometimes, perhaps even unique.
Macrophage response to foreign material
Contact of macrophages with foreign material, both in the form of small particles and in the form of extensive surfaces, leads to their activation. One of the serious problems in traumatology and orthopedics associated with a reaction to a foreign body is the development of joint instability after endoprosthetics, which, according to some data, is detected in 25–60% of patients in the first years after the operation and does not tend to decrease[36 ].
The surface of orthopedic prostheses wears out with the formation of particles that infiltrate the soft tissue. The chemical properties of the material determine the possibility of opsonization of particles by blood plasma proteins and the type of surface receptors that initiate phagocytosis. Thus, polyethylene, which activates complement, undergoes opsonization and is “recognized” by the complement receptor CR3, while titanium particles are absorbed by the cell through the opsonin-independent receptor MARCO. Phagocytosis of metal particles, synthetic polymers, ceramics, and hydroxyapatite by macrophages triggers the synthesis of proinflammatory mediators and the osteoclastogenesis inducer RANKL. CCL3 secreted by macrophages causes the migration of osteoclasts, and IL-1b, TNF-a, CCL5 and PGE2 stimulate their differentiation and activation. Osteoclasts resorb bone in the prosthetic area, but new bone formation is suppressed, since the corpuscular material inhibits collagen synthesis, inhibits the proliferation and differentiation of osteoblasts and induces their apoptosis [37, 38]. The inflammatory response caused by wear particles is considered the main cause of osteolysis.
Contact of tissues with material that cannot be phagocytosed initiates a cascade of events known as the foreign body response, or tissue reaction. It consists in the adsorption of plasma proteins, the development of an inflammatory response, initially acute, subsequently chronic, the proliferation of myofibroblasts and fibroblasts and the formation of a fibrous capsule that delimits the foreign body from the surrounding tissues. The main cells of persistent inflammation at the material/tissue interface are macrophages; its severity determines the degree of fibrosis in the contact zone. Interest in the study of tissue reactions is associated primarily with the widespread use of synthetic materials in various fields of medicine [12, 39, 40].
Adsorption of blood plasma proteins is the first stage of interaction of implanted materials with body tissues. The chemical composition, free energy, polarity of surface functional groups, and the degree of surface hydrophilicity determine the amount, composition and conformational changes in the bound proteins, which are the matrix for subsequent cell adhesion, including macrophages. The most significant in this regard are fibrinogen, IgG, complement system proteins, vitronectin, fibronectin and albumin.
A layer of fibrinogen quickly forms on almost all foreign materials. On hydrophobic surfaces, fibrinogen forms a monolayer of tightly bound, partially denatured protein, the epitopes of which are open to interaction with cellular receptors. On hydrophilic materials, fibrinogen is more often deposited in the form of a loose multilayer coating, and the outer layers are weakly or practically not denatured, leaving binding sites inaccessible to cellular receptors of macrophages and platelets[41, 42].
Many synthetic polymers have the ability to absorb components of the complement system and activate it with the formation of the C3-convertase complex. The fragments C3a and C5a generated by it are chemoattractants and activators of phagocytes, iC3b acts as a ligand for the cell adhesion receptor. The activation cascade can be triggered via both classical (mediated by adsorbed JgG molecules) and alternative pathways[43]. The latter is initiated by the binding of the C3 component to surfaces bearing functional groups, for example – OH-, causing its hydrolysis. The alternative pathway can also be switched on after the classical pathway or together with it due to the work of the C3 convertase of the classical pathway, which generates fragments of C3b, the triggering factor of the amplification loop, that are fixed on surfaces. However, sorption and even the beginning hydrolysis of C3 do not always lead to the appearance of an amplification signal. For example, C3 is strongly sorbed by polyvinylpyrrolidone, but its proteolysis on this surface is weakly expressed. Fluorinated surfaces, silicone and polystyrene weakly activate complement. For cellular reactions on foreign surfaces, not only the activation of the complement system is important, but the binding of other proteins mediated by its fragments is important.
The role of albumin lies in its ability to bind proteins of the complement system[12]. It does not promote the adhesion of macrophages and, unlike fibrinogen, does not induce their synthesis of TNF-a[44]. Fibronectin and vitronectin, proteins rich in RGD sequences (amino acid regions ARG-GLY-ASP), are usually found on implanted materials.
With regard to vitronectin, it is unknown whether it is adsorbed directly on the surface of the material or is part of the inactivated membrane attack complement complex fixed on it. Its significance for the development of tissue reaction is that it ensures the strongest and longest-lasting adhesion of macrophages. The interaction of macrophages with the substrate is ensured by cellular receptors for integrin proteins (avβ3, a5β1, CR3), rich in RGD sequences (Table). Blockade of macrophage adhesion by soluble RGD mimetics or removal of the CR3 receptor from their surface reduces the intensity of the tissue reaction, reducing the thickness of the forming fibrous capsule [12, 41, 45].
Attached macrophages fuse to form multinucleated cells (giant foreign body cells - GCTC). Inducers of this process are IFNγ, IL-1, IL-2, IL-3, IL-4, IL-13 and GM-CSF, which stimulate the expression of mannose receptors, which play an important role in cell fusion[12]. GKIT function as macrophages - they have the ability to phagocytose, generate oxygen and nitrogen radicals, synthesize cytokines and growth factors. The nature of the synthetic activity of these cells apparently depends on their “age”: at the early stages of the development of the tissue reaction, IL-1a, TNF-a are expressed, and later there is a switch to anti-inflammatory and profibrogenic mediators - IL-4, IL-10, IL-13, TGF-β[46, 47].
The response of macrophages to foreign materials has been studied under various conditions in vitro and in vivo. In in vitro experiments, the intensity of their adhesion on the surface under study and the formation of HCIT, the number of “turned on” genes, the number of synthesized and secreted enzymes, cytokines and chemokines are taken into account. In monocultures of mononuclear phagocytes adhered to various surfaces, it is not their polarization in the M1 and M2 directions that occurs, but the formation of mixed-type macrophages secreting both pro- and anti-inflammatory mediators with a shift towards the latter during long-term cultivation [12, 48, 49] . The absence of a “gold standard” - a stable control material that has proven itself when implanted into a living organism, with which the tested materials could be compared, as well as the use of non-standardized macrophage cell lines, different methods of their differentiation make it difficult to compare the results of the work of different authors. However, in vitro studies make it possible to judge the cytotoxicity of materials and determine the reaction of macrophages to their chemical modification. Valuable information was obtained by studying the activation of macrophages on the surface of various collagens - native and chemically modified. Native collagens induce in vitro the synthesis of signaling molecules by macrophages, both stimulating the inflammatory response (TNF-a, IL-6, IL-8, IL-1β, IL-12, CCL2) and suppressing it (IL-1ra, IL-10 ), as well as matrix metalloproteases and their inhibitors.[48-50]. The pro-inflammatory properties of such materials depend on the method of decellularization and sterilization of the starting material, which significantly changes its characteristics. Collagen endoprostheses obtained using different technologies from native collagen vary in their ability to induce the expression of proinflammatory cytokines from practically inert to highly active[51]. Injecting collagen with various chemicals changes the nature of the macrophage reaction. Treatment with glutaraldehyde leads to cytotoxicity, manifested in damage to the cytoplasmic membrane, impaired adhesion, and decreased viability of macrophages. At the same time, their production of IL-6 and TNF-a is increased, and the synthesis of IL-1ra is suppressed in comparison with macrophages adhered to native and carbodiimide-stitched collagen. Treatment with carbodiimide provides optimal properties to collagen, which is not cytotoxic, does not cause a significant increase in the secretion of proinflammatory cytokines and metalloproteases, and does not suppress the synthesis of IL-10 and IL-1ra in comparison with native [49].
In order to reduce the tissue reaction, components of the intercellular matrix, native or modified, are introduced into collagen materials. J. Kajahn et al. (2012) created an in vitro imitation of the pro-inflammatory microenvironment of endoprostheses, which promoted the differentiation of monocytes in the M1 direction[50]. Under the same conditions, additional sulfated hyaluronic acid introduced into the collagen substrate reduced the secretion of proinflammatory cytokines by macrophages and increased the production of IL-10. According to the authors, this indicates M2 polarization of macrophages, promoting regeneration and restoration of the functional properties of surrounding tissues. The response of macrophages to slowly degradable and stable materials in vitro is generally uniform and similar to the response to biomaterials, although some specificity of the response is still noticeable. Titanium, polyurethane, polymethyl methacrylate, polytetrafluoroethylene are weak inducers of inflammatory mediators, although titanium promotes higher secretion of TNF-a and IL-10 than polyurethane, and the peculiarity of polypropylene is to stimulate the production of the profibrogenic chemokine CCL18 [52-54]. PEG, proposed as a substrate for cell transfer, causes a sharp but rapidly increasing expression of IL-1β, TNF-a, IL-12, however, its copolymerization with cell adhesion oligopeptide improves the biocompatibility of the material, significantly reducing the expression of pro-inflammatory cytokines[55 ].
The response of macrophages to various materials in vitro does not fully characterize their behavior in the body. In monocultures, there are no factors of interaction with other cell populations and phenotypic polymorphism is not taken into account - in natural conditions, not only monocytic precursors migrate to the implant, but also mature tissue macrophages, the response of which can differ significantly from those recruited from the blood. The study of the secretory activity of macrophages surrounding endoprostheses installed in animal and human tissue is very difficult. The main method for characterizing macrophages based on the M1-M2 paradigm in situ was data from immunocytochemistry of the marker proteins iNOS, CD206, CD163, CD80, CD86. It is postulated that the presence of these markers in macrophages in vivo determines their polarization in the M1 and M2 directions with the synthesis of the corresponding spectra of cyto- and chemokines, but, given the possibility of the existence of mixed type macrophages [34], this characteristic is not entirely correct.
However, in vivo experiments make it possible to trace the fate of the implanted material and the dynamics of the macrophage response over a long period, which is especially important for life-long endoprostheses and devices. The most studied in this aspect are degradable biomaterials based on collagen. The first inflammatory cells to migrate to such materials are PMNs, but this effect is transient and the second wave population is represented by macrophages[56, 57]. Their reaction depends on the physicochemical properties of collagen. The harsher the chemical treatment, the more the collagen differs from the native one, the more “foreign” it becomes for the macrophage and the more pronounced the tissue reaction. Fragments of implants made of slowly degrading stitched collagen installed between the muscle layers of the abdominal wall of a rat promote the formation of GCI and material encapsulation. Migrating macrophages, judging by the expression of the CCR7 and CD206 receptors, can be attributed in some cases to the M1 phenotype, but in many cases it is not possible to determine their belonging to the known phenotypes.
Over time, M2 macrophages appear around the implant, which are located predominantly in the fibrous capsule[58]. Endoprostheses made from non-stitched pig, human and bovine collagen[58] and diisocyanate-stitched sheep collagen[59], which are quickly destroyed in the rat’s body, stimulate the new formation of full-fledged connective and muscle tissue. They do not contribute to the formation of HCIT and are not encapsulated. Some mononuclear phagocytes accumulating at the tissue/material interface do not have M1/M2 phenotype markers, some contain both markers, and some are M2 macrophages. The M1 macrophage subpopulation is absent on such implants[58–60]. Histomorphometric analysis showed a positive correlation between the number of macrophages carrying markers of the M2 phenotype in the early stages of the developing tissue reaction and indicators of successful tissue remodeling in the implantation zone[58].
The tissue reaction to non-degradable materials exists throughout the entire time of their presence in the body[61]. Its intensity is modulated by the physicochemical properties of the materials: in the series polyester, polytetrafluoroethylene, polypropylene - the first polymer causes the most pronounced inflammation and fusion of macrophages, the last - minimal, and the severity of fibrosis for all of these materials positively correlates with the amount of HCIT on the surface of synthetic polymers[40] . Despite the large number of studies that have studied the inflammatory response to various materials, the characteristics of macrophages accumulating on them have not been sufficiently studied. M. T. Wolf et al. (2014) showed that predominantly macrophages with markers of the M1 phenotype (CD86+CD206-) accumulate on the threads and between the nodes of a polypropylene mesh implanted into the abdominal wall of a rat [57].
A gel from the intercellular matrix of connective tissue applied to polypropylene reduces the number of M1 macrophages and GCT and at the same time inhibits the growth of microvessels. This phenomenon is in good agreement with the results of studies demonstrating the expression of M1 angiogenic factors by wound macrophages and the suppression of vasculogenesis during their blockade [21, 52]. Little is known about the synthetic activity of macrophages and the spectrum of their biologically active molecules that provide tissue reactions. In a mouse, macrophages secreting IL-6 and CCL2, IL-13 and TGF-β accumulate at the periphery of the nylon mesh implantation zone, and at the same time, IL-4 is expressed in the cell population, including in the GCIT, adhered to the fibers of the endoprosthesis , IL-10, IL-13 and TGF-β[47]. IL-4 and IL-13 are powerful profibrogenic mediators; they not only polarize macrophages in the M2a direction, promoting the production of growth factors, but also, through the induction of TGF-β expression by fibroblasts, stimulate their collagen synthesis. IL-10 and CCL2 also have a profibrogenic effect, providing chemotaxis of myofibroblast precursors—fibrocytes[29, 30, 62, 63]. It can be assumed that it is macrophages that create an environment conducive to the development of fibrosis around non-degradable materials.
The formation of fibrous tissue can have both negative and positive effects on patient outcomes. In herniological practice, fibrous tissue transformation associated with the implantation of a polypropylene endoprosthesis is one of the main problems (Fig. 2, own data), which, against the background of irrational surgical tactics, in 15–20% of cases leads to the development of recurrent hernias of various localizations [39, 64 ].
In recent years, dental implantation technologies based on the integration of installed structures through the development of connective tissue have been developing especially intensively (Fig. 3, own data). Despite the fact that fibrointegration of implants is recognized by a number of specialists as a valid option, the search for new materials that promote osseointegration processes continues[65].
In this regard, the study of cell populations in the prosthetic area, the development of methods and approaches to blocking an excessive inflammatory reaction leading to fibrosis and stimulating reparative regeneration at the site of implantation of various materials are of significant importance.
Conclusion
Macrophages are a polymorphic population of cells whose phenotype is determined by microenvironmental signals. They play a decisive role in the body’s response to foreign material used for endoprosthetics, catheterization, stenting and other types of treatment. The nature of the reaction and the degree of its severity depend both on the size of the implanted material and on its physicochemical properties and can have both positive and negative implications for the patient’s body. For degradable collagen-based materials, the dependence of the type of macrophage activation and the rate of connective tissue regeneration on the method of processing collagen raw materials has been shown. This opens up great opportunities for specialists developing new methods for tissue decellularization, chemical modification and sterilization of collagen materials in order to obtain implants for regenerative medicine.
Problems associated with the activation of macrophages by non-degradable materials, apparently, should be solved differently. Macrophages phagocytizing wear microparticles on the surface of joint endoprostheses and macrophages migrating to the extensive surfaces of synthetic implants initiate long-term persistent inflammation, osteolysis in the first case and fibrosis in the second. Mitigation of this effect will most likely be achieved by blocking directional migration, adhesion and activation of monocytes/macrophages, which will require deeper knowledge of these processes than we currently have.