In the blood, lymphocytes are part of the leukocyte formula (20-35%), essentially represented by different populations depending on immune function. They are formed in the bone marrow from table lymphoid precursor cells. A common property of all lymphocytes is their participation in immune tolerance – the ability of the immune system to recognize and not attack its own tissues. To this end, cells that encounter their antigens during maturation are eliminated. The growth, development and work of lymphocytes are closely related to special molecules, which we will consider in articles later, gradually introducing their designations.
“Combat” and “self-controlling” functions of the immune system
For more information see:
Note. This page briefly reviews the functions and characteristics of the main immunocompetent cells: T and B lymphocytes and dendritic cells (DCs). But at the beginning, a characteristic is given of the special dual functionality of the immune system, in which lymphocytes play the main role. Therefore, it is worth recalling what these cells are.
Lymphocytes are cells of the immune system, which are a type of white blood cell that provide humoral immunity (production of antibodies), cellular immunity (contact interaction with target cells), and also regulate the activity of other types of cells. Based on morphological characteristics, two types of lymphocytes are distinguished: large granular lymphocytes (most often they are NK cells and small lymphocytes (T cells and B cells).
Types of Lymphocytes
Almost all animals have some type of immune defense mechanism. These mechanisms vary greatly in their structure, complexity, efficiency and, most importantly, in the ratio of innate and acquired components. In invertebrates, innate immunity predominates, although this is far from an absolute rule. In addition to innate protective mechanisms, vertebrates have developed an unusually complex adaptive immune system, capable of adapting to all sorts of new infections, developing new means of combating them, and also having a good memory (it is thanks to immune memory that we receive lasting immunity to many diseases, once having had them).
In all “higher” vertebrates, the main components of the adaptive immune system are two types of lymphocytes: B and T (see: B cell, T cell). Each mature lymphocyte produces one (and only one) type of receptor, and each receptor is capable of recognizing foreign molecules (antigens) of a strictly defined type. B-lymphocyte receptors are called antibodies; they can detach from the surface of the lymphocyte and independently attack “enemies” (for example, bacteria). T-lymphocyte receptors (T-cell receptors) are similar in structure to antibodies, but they are firmly attached to the surface of the T-lymphocyte and do not diffuse freely into the environment like antibodies.
During the development (maturation) of lymphocytes, a complex restructuring of their genome occurs. Its essence is that from the set of “blanks” available in the genome, mature, ready-to-use antibody or T-cell receptor genes are formed in a combinatorial manner. A huge variety of lymphocytes arises, producing hundreds of thousands and millions of different immune receptors. Among these receptors, those dangerous to the body inevitably appear, ready to attack their own antigens. Lymphocytes that produce such receptors are rejected; the rest are retained. As a result, the body receives a huge set of lymphocytes capable of recognizing almost any foreign proteins and carbohydrates. When an infection (for example, bacteria) enters the body, those B-lymphocytes whose antibodies exhibit the greatest affinity for the surface substances (antigens) of a given bacterium additionally “adjust” their antibody genes to these antigens through somatic hypermutation.
T- and B-lymphocytes of higher vertebrates exchange a variety of chemical signals with each other (see interleukins below). In accordance with this, strictly defined genes responsible for receiving and transmitting these signals are active in different types of lymphocytes.
Interleukins are a group of cytokines (proteins) synthesized and secreted by T lymphocytes, B lymphocytes and NK cells, as well as cells interacting with them. Cytokines are small peptide information molecules. Cytokines have a molecular weight not exceeding 30 kD. The cytokine is released onto the surface of cell A and interacts with the receptor of the nearby cell B. Thus, from cell A to cell B , which triggers further reactions in cell B. Their main producers are lymphocytes (for more information about cytokines, see here →).
For more information on cytokines, see the separate section:
One of the main functional differences between B and T lymphocytes is that the former specialize primarily in fighting infections through the mass production of antibodies, while the latter are more “scouts” and specialists in finely distinguishing between “friends” and “foes.” » antigens; in particular, they watch carefully to ensure that B lymphocytes do not mistakenly start producing autoimmune antibodies that attack the body's own cells.
T-cell receptors identify the presence of a foreign protein molecule only if this molecule has already entered some other cell of the body (for example, a B-lymphocyte) and has been broken down into short pieces there. These pieces are then combined with special proteins (they are called proteins of the Major Histocompatibility Complex (MHC, major histocompatibility complex). Cells exhibit complexes of MHC proteins and short fragments of other proteins - their own and others - on their surface specifically in order to T-lymphocytes could “feel" them with their receptors. T-cell receptors accurately distinguish “self” from “foreign,” but only if the test sample is carefully cut into pieces of the required length and attached to the MHC protein.
The division of labor between lymphocytes inevitably follows from the very “idea” of producing a huge number of diverse immune receptors in a combinatorial manner. On the one hand, thanks to this technology, vertebrates are more successful than any other animals in resisting various infections. It is unlikely that without this our ancestors would have been able to become warm-blooded, that is, turn into a walking thermostat with a breeding ground for microbes. On the other hand, such an immune system must be under strict control, otherwise it will instantly kill its own body. Who can control which antigen is recognized by this or that lymphocyte, whether this antigen is definitely foreign, and whether there will be any harm to the body if this lymphocyte begins to multiply? Apart from another lymphocyte, no one will understand this. The immune system must control itself.
It is very difficult to combine both functions - “combat” and “self-control” - in the same cell, especially if these functions must be performed with very high efficiency. This creates the prerequisites for the division of labor between lymphocytes: some of them specialize in fighting infection, while others closely monitor the first ones so that they do not mistakenly begin to produce antibodies dangerous to the body.
Patient preparation rules
Standard conditions:
Fasting period of 8 hours (unless otherwise determined by the doctor). You can drink water. Biomaterial is accepted in accordance with the schedule for taking biomaterial in the ML department of DILA. On the eve of the study, it is necessary to exclude physical activity (sports training) and smoking. Children under 5 years of age and adults with contraindications for fasting are allowed a light meal of low-fat food at least 2 hours before the BM test.
You can add this study to your cart on this page
Immune activation and dendritic cells
Source: “Science and Life” No. 11, 2011 Author: Olga Belokoneva, Candidate of Chemical Sciences
In 2011, the Nobel Prize in Physiology or Medicine was awarded to Bruce Beutler and Jules Hoffmann for their discovery of the mechanisms of activation of innate immunity, and to Ralph Steinman for the discovery of dendritic cells and their role in the activation of adaptive immunity. These studies are called revolutionary because the discovery of innate immunity radically changed the understanding of the functioning of the immune system.
Immunity . This word has long and firmly taken its place in the vocabulary of modern man. Most often, immunity refers to the body’s ability to protect itself from dangerous viruses, bacteria, fungi or other parasites. But what kind of mechanism this is and how, in fact, this protection is carried out, only a few specialists understand. The mechanism is indeed very complex. This year's Nobel laureates revealed only some of the key aspects of the “first line of defense” - the innate immune system.
In nature, there are two lines of defense, two types of immunity.
The first and most ancient is the innate immune system, which is aimed at destroying the cell membrane of a foreign cell. It is inherent in all living beings - from fruit flies to humans. If, however, any foreign protein molecule manages to break through the “first line of defense,” it is dealt with by the “second line”—adaptive, or acquired, immunity.
Adaptive immunity is the highest form of defense that is unique to vertebrates. The mechanism of acquired immunity is very finely tuned and specific. In short: when a foreign protein molecule enters the body, white blood cells (leukocytes) begin to produce antibodies - for each protein (antigen) its own specific antibody is produced. First, the so-called T cells (T lymphocytes) are activated, which begin to produce active substances, cytokines, that trigger the synthesis of antibodies by B cells (B lymphocytes). The strength or weakness of the immune system is usually assessed by the number of B and T cells, so important are they for protecting the body. The antigen-antibody interaction is very strong and very specific. When antibodies “sit” on antigen proteins located on the surface of a virus or bacteria, the development of infection in the body is blocked ( for more information about adaptive immunity, see here → ).
The process of antibody production does not start immediately; it has a certain incubation period, depending on the type of pathogen. But, if the activation process has already begun, as soon as the same infection tries to enter the body again, the B-cells will instantly respond by producing antibodies, and the infection will be destroyed immediately, without causing any harm. That is why a person develops immunity to certain types of infections for the rest of his life.
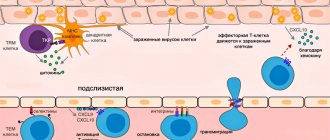
Figure 1. Dendritic cells show T cells their “enemies” . Toll
-like receptors on the surface interact with characteristic molecular structures on the surface of the bacterial cell membrane (PAMP), a biochemical signal is sent into the cell, and antigens are brought to the surface of the dendritic cell, which T cells can easily “feel” using special antigen-recognition proteins.
“Recognition” of the antigen is accompanied by the activation of T cells, followed by their transformation into T helper cells and the launch of a cascade of biochemical reactions, the final result of which is the production of specific antibodies by B cells. In addition, under the influence of PAMP, dendritic cells and macrophages produce special molecules - cytokines, which also contribute to the activation of T cells. Adapted figure from the article Medzhitov R. Toll-like receptors and innate immunity. Nat.
Rev. Immunol . 2001, 1, 135–142.
But the innate immune system is nonspecific and does not have “long-term memory”, since it reacts to certain molecular structures inherent in all pathogenic microorganisms. These structures are called pathogen-associated molecular patterns
- PAMP). These PAMPs are molecules that are part of the bacterial cell membrane. Despite the chemical differences, all these structures have the following properties: they are synthesized only by microorganisms (they are not found in animal cells, so recognition of PAMPs is regarded by the immune system as a signal to start fighting the invader); they are characteristic of a number of pathogens, and not just one; These structures are important for the life of the bacterium, so during the process of evolution they change very slowly (otherwise the immune system simply would not have time to set up recognition). If bacteria manage to break through the “first line of defense” and avoid destruction by macrophages or granulocytes, then the acquired immune system must join the fight.
How does the innate immune system signal the acquired immune system to produce specific antibodies? It was for the solution to this key question in immunology that the 2011 Nobel Prize was awarded.
In 1973, Ralph Steinman discovered a new type of cell, which he called dendritic, because in appearance they resembled the dendrites of neurons. Cells were found in all tissues of the body that came into contact with the external environment: in the skin, lungs, and the mucous membrane of the gastrointestinal tract. First, the researcher suggested (at that time this caused skepticism of many scientists), and then proved that dendritic cells serve as intermediaries between innate and acquired immunity. That is, the “first line of defense” sends a signal through them that activates T cells and triggers a cascade of antibody production by B cells ( for more information about innate immunity, see here → ).
Dendritic cells _ _
, DC) is a population of special cells of the immune system of bone marrow origin, the function of which is to present “enemy” antigens to other cells of the immune system.
In this way they activate adaptive immunity. Scientifically, such messenger cells are called antigen presenting cells ( APCs ).
See mechanism of action in fig. 1 and fig. 2). As it turned out later, dendritic cells (as well as macrophages and epithelial cells) have special protein complexes on their cell surface - receptors. The genes encoding these receptors are similar to Toll
-genes of the fruit fly Drosophila (from German
toll
- stunning, crazy), playing a key role in embryogenesis.
In 1996, Jules Hoffmann discovered that flies with the Toll
gene “switched off” had no immunity at all and died from any fungal infection.
Hoffmann suggested that the Toll
is important not only for the development of the embryo, it also plays a key role in the immune system.
As it turns out, this gene encodes special receptors that recognize molecules in the membrane structure of bacterial pathogens (PAMPs), sending a biochemical signal to eliminate the “intruder.” They were called “ Toll
like
receptors” .
When PAMP interacts with Toll
-like receptor proteins appear on the surface of the dendritic cell, which trigger the adaptive immune response of T cells.
Toll
have been found in humans . Some of them are located on the surface of cells, others “float” in the cell cytoplasm. The end result of PAMP interaction with these receptors is T cell activation. At the cellular level, phagocytes are activated: they begin to produce reactive oxygen species, and therefore, more intensively digest “scraps” of the cell walls of foreign bacteria.
In 1998, Bruce Beutler studied the receptors of bacterial lipopolysaccharides (LPS), molecules in which a lipid and a sugar are “cross-linked” together. LPS are immunologically very active molecules; they not only stimulate, but “superstimulate” the immune system, causing septic shock under certain conditions. Beutler tried to find the gene responsible for the effects of LPS and discovered that mice insensitive to LPS had a mutation in a gene very similar to Toll
- Drosophila fly gene.
The Toll
-like receptor accidentally turned out to be the elusive LPS receptor, that is, LPS interacts with
the Toll
-like receptor, leading to the activation of inflammatory processes, including septic shock. It turned out that flies and mice have the same mechanism of protection against infection. The “harmful” components of the membrane of cellular bacteria, which caused the innate immune response, turned out to be lipopolysaccharides - components of the cell wall of gram-negative bacteria.
Thus, the discovery of innate immunity has led to the emergence of new approaches in the prevention and treatment of diseases, in the development of new vaccines and antitumor drugs.
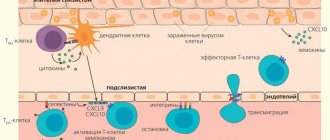
Activation of the adaptive immune system
Adaptive immunity can differentiate between specific pathogens and target a response that is specific to a given pathogen. It can respond quickly upon repeated exposure to a particular pathogen, preventing the development of symptoms (immunological memory). The adaptive immune system is coordinated by lymphocytes (a class of white blood cells) and results in the production of antibodies. B lymphocytes (B cells) are antibody-producing cells that recognize and target a specific fragment of a pathogen ( antigen ). Helper T lymphocytes (T cells) are regulatory cells that release chemicals (cytokines) to activate specific B lymphocytes
- When phagocytic leukocytes engulf a pathogen, some of them present digested fragments (antigens) on their surface
- These antigen presenting cells (dendritic cells) migrate to the lymph nodes and activate specific helper T cells
- Helper T cells then release cytokines to activate the specific B cell, which can produce antibodies specific to the antigen
- The activated B cell will divide and differentiate to form short-lived plasma cells that produce large amounts of specific antibodies
- Antibodies will target their specific antigen, enhancing the immune system's ability to recognize and destroy the pathogen.
A small proportion of activated B cells (and activated TH cells) will develop into memory cells to provide long-lasting immunity.
See more:
Lymphocytes are responsible for acquired immunity
How to distinguish resident tissue cells from admixtures of blood cells?
Resident T cells can be correctly, but inconveniently, determined each time by the ability of an individual cell to migrate to lymph nodes, so it is necessary to compile a list of characteristic features that can be used to determine membership in this subpopulation. Resident T lymphocytes in the body's natural barrier tissues (for example, in the lungs and small intestinal mucosa) are somewhat similar to classical effector blood cells: they express the activated cell marker CD69, and the expression is stable throughout life during adulthood and aging and is characteristic of all non-lymphoid tissues . But in addition, CD69 colocalizes with the marker CD103, which denotes a group of adhesion molecules - integrins, which promote the attachment of resident T cells to the epithelium and to fibroblasts in the submucosa of the selected organ. For effector T cells in secondary lymphoid organs, the expression of CD103 integrins is completely uncharacteristic: TEM cells constantly maintain a motile phenotype.
The map compiled by Donna Farber's team has a major flaw: it is unclear how cleanly T-lymphocytes can be isolated from an organ, and what proportion of the analyzed cells are actually blood T-lymphocytes from capillaries inside the organ.
The issue of contamination with blood cells is especially acute for the lungs; it is no coincidence that the subpopulation composition of T cells in the lungs is unexpectedly similar to T cells in the blood and lymph nodes. The issue of blood cell contamination was elegantly solved for mouse T cells: experimental mice were infected with lymphocytic choriomeningitis virus after transplantation of a transgenic P14 T cell clone specific for this virus. As a result, during infection, the majority of circulating cells were represented by the virus-specific P14 clone, and its presence in tissues could be monitored by immunofluorescence with a P14-specific antibody. Before the mice were killed, they were injected with an antibody to the killer T-cell marker anti-CD8, which quickly spread through the bloodstream and bound to all killer T-cells in the blood (but not in the tissues). By microscopy of organ sections, it was easy to distinguish resident killer TRMs from cells only recently released from the blood into the organ, labeled with an anti-CD8 antibody [7]. The number of resident cells calculated by this method was 70 times higher than the numbers determined by flow cytometry; a difference of less than twofold was observed only for resident cells of the lymph nodes and spleen: it turns out that standard methods for isolating lymphocytes from organs are poorly suited for the analysis of killer resident cells and significantly underestimate the population size.
1. General characteristics of B-lymphocytes
B lymphocytes ( B cells ) are a functional type of lymphocyte that plays an important role in providing humoral immunity. When exposed to antigen or stimulated by T cells, some B lymphocytes transform into plasma cells capable of producing antibodies. Other activated B lymphocytes become memory B cells. In addition to producing antibodies, B cells perform many other functions: they act as antigen-presenting cells and produce cytokines and exosomes.
B lymphocytes produce and secrete antibody molecules into the bloodstream, which are modified forms of the antigen recognition receptors of these lymphocytes . The appearance of antibodies in the blood after the appearance of any foreign antigen protein, regardless of whether it is harmful or harmless to the body, is an immune response. The appearance of antibodies is not just a protective reaction of the body against infectious diseases, but a phenomenon that has broad biological significance: it is a general mechanism for recognizing “foreign”. For example, the immune reaction will recognize as foreign and will try to remove from the body any abnormal and, therefore, potentially dangerous variant of the cell in which, as a result of a mutation in the chromosomal DNA, a mutant protein molecule is formed.
Mammalian B lymphocytes differentiate first in the fetal liver and, after birth, in the red bone marrow. The cytoplasm of resting B cells lacks granules but contains scattered ribosomes and rough endoplasmic reticulum tubules. Each B cell is genetically programmed to synthesize immunoglobulin molecules embedded in the cytoplasmic membrane. Immunoglobulins function as antigen recognition receptors specific for a particular antigen. About one hundred thousand receptor molecules are expressed on the surface of each lymphocyte. Having encountered and recognized an antigen that matches the structure of the antigen recognition receptor, B cells multiply and differentiate into plasma cells, which form and secrete in soluble form large quantities of such receptor molecules - antibodies. Antibodies are large glycoproteins found in blood and tissue fluid. Due to their identity with the original receptor molecules, they interact with the antigen that originally activated the B cells, thus exhibiting strict specificity.
Once the antigen binds to the B cell's receptors, the cell becomes activated. B cell activation consists of two phases: proliferation and differentiation; all processes are induced by contact with antigen and T-helpers.
As a result of proliferation, the number of cells capable of reacting with the antigen introduced into the body increases. The importance of proliferation is great because in a non-immunized organism there are very few B cells specific for certain antigens.
Some of the cells that proliferate under the influence of the antigen mature and differentiate sequentially into antibody-forming cells of several morphological types, including plasma cells. Intermediate stages of B cell differentiation are marked by the changing expression of a variety of cell surface proteins required for B cell interactions with other cells.
Each B-lymphocyte that differentiates in the bone marrow is programmed to produce antibodies of only one specificity.
Antibody molecules are not synthesized by any other cells of the body, and all their diversity is due to the formation of several million clones of B cells. They (antibody molecules) are expressed on the surface membrane of the lymphocyte and function as receptors. At the same time, about one hundred thousand antibody molecules are expressed on the surface of each lymphocyte. In addition, B lymphocytes secrete into the bloodstream the antibody molecules they produce, which are modified forms of the surface receptors of these lymphocytes.
Antibodies are formed before the antigen appears, and the antigen itself selects antibodies for itself. As soon as an antigen enters the human body, it literally encounters an army of lymphocytes carrying various antibodies, each with its own individual recognition site. The antigen binds only to those receptors that exactly match it. Lymphocytes that have bound the antigen receive a trigger signal and differentiate into plasma cells that produce antibodies. Since the lymphocyte is programmed to synthesize antibodies of only one specificity, the antibodies secreted by the plasma cell will be identical to their original, i.e. surface receptor of the lymphocyte and, therefore, will bind well to the antigen. So the antigen itself selects antibodies that recognize it with high efficiency.
Literature
- Clark RA (2010). Skin resident T cells: the ups and downs of on site immunity. J. Invest. Dermatol. 130 (2), 362–370;
- Husband and wife, one of Satan?;
- Iijima N. and Iwasaki A. (2015). Tissue instruction for migration and retention of TRM cells. Trends Immunol. 36, 556–564;
- Schenkel J. M. and Masopust D. (2014). Tissue-resident memory T cells. Immunity. 41, 885–897;
- Thome JJ, Yudanin N., Ohmura Y., Kubota M., Grinshpun B., Sathaliyawala T. et al. (2014). Spatial map of human T cell compartmentalization and maintenance over decades of life. Cell. 159, 814–828;
- Where did vision come from?
- Steinert EM, Schenkel JM, Fraser KA, Beura LK, Manlove LS, Igyártó BZ et al. (2015). Quantifying memory CD8 T cells reveals regionalization of immunosurveillance. Cell. 161, 737–749;
- Frost EL, Kersh AE, Evavold BD, Lukacher AE (2015). Cutting edge: resident memory CD8 T cells express high-affinity TCRs. J. Immunol. 195, 3520–3524;
- Park CO and Kupper T S (2015). The emerging role of resident memory T cells in protective immunity and inflammatory disease. Nat. Med. 21, 688–697;
- Schluns KS and Klonowski K. Diverse functions of mucosal resident memory T cells. E-book, 2015;
- Godfrey DI, Uldrich AP, McCluskey J, Rossjohn J, Moody DB (2015). The burgeoning family of unconventional T cells. Nat. Immunol. 16, 1114–1123;
- Castellana D., Paus R., Perez-Moreno M. (2014). Macrophages contribute to the cyclic activation of adult hair follicle stem cells. PLoS Biol. 12 , e1002002;
- Rodero M. P. and Khosrotehrani K. (2010). Skin wound healing modulation by macrophages. Int. J. Clin. Exp. Pathol. 3 (7), 643–653;
- Farber D., Yudanin N., Restifo N. P. (2014). Human memory T cells: generation, compartmentalization and homeostasis. Nat. Rev. Immunol. 14, 24–35..