The essence of the Dopplerography method of uteroplacental blood flow
The newly formed fetal blood supply system supplies nutrition not only to the unborn baby, but also to the placenta - the protective membrane. Not only the physical protection of the fetus depends on the completeness and timeliness of blood supply. Good blood supply affects:
- the ability of the placenta to prevent the penetration of toxins;
- the baby's development in the following weeks;
- the ability of the mother's body to neutralize toxins.
Doppler ultrasound (DPM) is a study using a conventional ultrasound machine. The essence of the technique is to use the Doppler effect: when reflected from moving objects (red blood cells), ultrasound waves change their frequency. The device records the received data and displays an image of the blood flow on the screen. An experienced doctor, based on research, determines whether there are deviations in the functioning of the blood supply system, and how dangerous they are.
Blood flow disorders during pregnancy
General information
Blood flow is responsible for the movement of blood through the vessels of the circulatory system. During pregnancy, blood flow in the vessels of the unborn baby, the umbilical cord, and the uterus is examined. Fetal Doppler is a subtype of ultrasound diagnostics that allows you to evaluate the characteristics of blood flow in the vessels of the child, uterus and placenta. Based on this study, the doctor can judge whether the baby is suffering from a lack of oxygen. The doctor can find out information about at what level the vascular pathology occurred (in the uterus, placenta or umbilical cord). Doppler measurements are carried out together with ultrasound.
Vascular examination during pregnancy
Using Doppler ultrasound , it is possible to assess blood flow in the fetal vessels. Doctors examine:
- aorta;
- pulmonary trunk;
- carotid arteries;
- arteries of the brain;
- liver vessels.
The most valuable information is about blood flow in the uterine arteries and umbilical cord arteries. Examining these vessels. The doctor learns about how the placenta develops. You can also find out whether the fetus has enough oxygen and nutrients.
Blood flow disorders during pregnancy
Impaired blood flow is characterized by an increase in the diastolic component of the blood flow rate. An increase in cerebral blood flow is a compensatory centralization of fetal circulation during intrauterine hypoxia. In the fetus, hypoxia is characterized by the redistribution of blood to the blood supply to vital organs:
- cerebral hemispheres;
- myocardium;
- adrenal glands
Characteristic of an asymmetric form of fetal growth retardation.
Increased ASC is also a pathological sign. An increase in SDO in the internal carotid artery may mean:
- intrauterine infection;
- pathology of the central nervous system.
Based on an analysis of the results obtained, many pregnant women were prescribed timely therapy: both medication and lifestyle correction. This helped keep the desired pregnancy healthy.
Causes of blood flow disorders during pregnancy
- circulatory disorders in the uterus can be caused by:
- increased blood pressure;
- pneumonia;
- intrauterine infection;
- hypoxia.
For correct diagnosis in obstetric practice, three-dimensional ultrasound images are used. With the help of this modern diagnostic method, there is a prospect of diagnosing retroplacental bleeding and assessing cardiac malformations by monitoring blood flow. The method is indispensable, as it helps to see devections even in the smallest vessels.
Treatment and prevention of blood flow disorders during pregnancy
In the modern world, there are opportunities for early detection of obstetric complications, so correction can be made at an early stage, avoiding problems with blood circulation in the future. A pregnant woman should remember that her emotional states are transmitted to the child. In order for the fetus to develop without complications, it is necessary to create the correct diet with a maximum of vitamins, macroelements, carbohydrates, proteins and fats. If a pregnant woman is not bothered by swelling, then fluid intake should be at least 1-1.5 liters. in a day.
It is important to monitor changes in body weight, since by the end of pregnancy the weight gain should not exceed 10 kg. There are risk groups that require the use of drug prophylaxis, which promotes the interaction of the body systems of the fetus and mother and prevents dysfunction of the uteroplacental circulation. Timely adjusted methods of labor management and drug therapy will help to significantly reduce perinatal morbidity and mortality . But a high risk of severe neurological complications cannot be ruled out.
Indications and contraindications for diagnostics
The basis for Dopplerography of uteroplacental blood flow are several groups of factors:
- Diseases of a pregnant woman: diabetes mellitus, hypertension, collagen vascular diseases, preeclampsia (increased blood pressure after 20 weeks), kidney disease, Rh sensitization (Rh difference between spouses).
- Diseases and malformations of the fetus: growth retardation, causeless oligohydramnios or dropsy, premature ripening of the placenta, discrepancy between fetal weight and gestational age, heart defects, etc.
- Other reasons: abdominal injuries, age more than 35 or less than 20 years, pathological type of cardiotocogram, postmaturity, unsuccessful previous pregnancies, entanglement of the fetal neck with the umbilical cord.
The doctor has the right to prescribe DPM for any condition of the pregnant woman or fetus that causes fear for the life of the unborn child. The study is used to assess the effectiveness of treatment for uteroplacental insufficiency and the threat of late miscarriage.
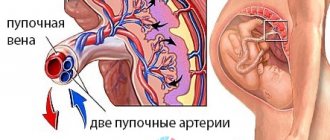
Why can a single umbilical cord artery be identified in a fetus?
Possible reasons for identifying one umbilical cord artery:
- Sometimes a single umbilical cord artery is identified in completely normal fetuses . After the birth of a child, this fact does not have any impact on its further development.
- Sometimes a single umbilical cord artery is combined with defects of the fetal cardiovascular system , therefore, when a single umbilical cord artery is identified, a detailed examination of the anatomy of the fetus and, in particular, the cardiovascular system is carried out. In the absence of other malformations, a single umbilical cord artery is able to provide adequate blood flow to the fetus.
- Somewhat more often, a single umbilical cord artery is detected in fetuses with Down syndrome and other chromosomal diseases . However, this marker is a “minor” marker of Down syndrome, so identifying only a single umbilical cord artery does not increase the risk of Down syndrome and is not an indication for other diagnostic procedures.
- A single umbilical cord artery sometimes results in intrauterine growth restriction . In this regard, if a single umbilical cord artery is detected, an additional ultrasound is recommended at 28 weeks of pregnancy, and a planned one at 32-34 weeks. If a lag in the size of the fetus from the gestational age or disruption of blood flow in the vessels of the fetus and uterus is not detected, then the diagnosis of fetal growth retardation is excluded.
Features of Doppler testing
The examination procedure is absolutely safe and is almost no different from a regular ultrasound:
- The patient lies on the couch on her back and exposes the abdominal area.
- The doctor applies a special gel to the pregnant woman’s skin to improve image quality.
- The specialist first examines the general condition of the uterus and fetus for possible abnormalities.
- After this, the doctor turns on the Doppler function and examines the condition of the area of \u200b\u200bthe circulatory system of interest: the aorta, cerebral arteries, etc.
- The results are automatically entered into a special section of the program and analyzed.
If there are deviations, this is immediately visible on the screen. The duration of the procedure is from several minutes to half an hour. The time depends on the volume of the area requiring inspection and the level of qualification of the specialist.
There are no contraindications to the procedure - this is a safe ultrasound examination method, which has been confirmed by many years of clinical trials.
Fetal growth restriction syndrome (FGR) is a serious complication of pregnancy and significantly increases the risk of stillbirth and perinatal mortality. It has been proven that children born with FGR have an increased likelihood of developing obesity, metabolic syndrome, hypertension and other cardiovascular diseases in later life [1]. The theoretical and experimental substantiation of this effect of FGR formed the basis of the theory of so-called intrauterine programming, which suggests that malnutrition of the fetus triggers compensatory mechanisms that ensure its survival in the womb, but at the same time program an earlier onset of the listed diseases [2-4]. Such programming is observed during maternal starvation, protein deficiency in her diet, maternal cardiovascular diseases, and in pregnant women living in high mountain areas under conditions of chronic hypoxia [5, 6]. However, disruption of fetoplacental circulation and deterioration of transplacental transport of nutrients play an almost major role in limiting the delivery of nutrients and oxygen to the fetus.
One of the important ultrasound markers of increased vascular resistance of the placenta is a decrease in the diastolic component of blood flow in the umbilical cord artery (AC), in critical cases becoming zero or even negative (directed from the placenta to the fetus) [7-9]. Existing drug approaches to the treatment of FGR against the background of zero and negative blood flows in the AP have shown to be ineffective, which led to the conclusion that it is impossible to correct true placental insufficiency [10, 11].
Assessment of blood flow in the umbilical cord artery against the background of FGR
Doppler study of blood flow velocity in the AP is currently a mandatory condition for the diagnosis and severity of FGR [12]. Early studies [13] conducted on chicken embryos and experimental animals showed that embolization of cotyledon vessels along the AP with glass microspheres is accompanied by an increase in vascular resistance and an increase in the systolic-diastolic ratio of blood flow velocities (S/D) in the AP. As the placenta develops during normal pregnancy, there is a decrease in its vascular resistance and a decrease in S/D [14–16]. In the case of FGR, as the gestational age increases, the decrease in S/D is significantly less [17]. Morphological studies of placentas have shown a close correlation between microscopic changes in the vessels of the placental villi and hemodynamic parameters in FGR [18–21].
A meta-analysis based on a significant number of clinical studies [22, 23] showed that systematic Doppler assessment of the uteroplacental circulation in high-risk pregnant women with FGR reduces the likelihood of perinatal losses by 29-38% without increasing the incidence of operative delivery in preterm pregnancy. pregnancy. Thus, further study of the mechanisms of regulation of fetoplacental blood circulation opens up new prospects for improving the outcomes of pregnancies complicated by FGR.
Vascular network of the placenta in FGR
It has been proven that hypoperfusion of the placenta on the maternal side is a significant factor in the occurrence of FGR [24, 25]. However, an equally important component is the actual state of the blood vessels of the placenta. Moreover, in approximately 60% of cases of FGR development, blood flow in the uterine arteries was not changed [26]. Morphological examination of placentas in the presence of fetuses with growth retardation showed a decrease in the size of the placenta itself, a high percentage of villi that do not have the corresponding vessels, fibrinoid necrosis of areas of the placenta and multiple villous infarctions [27, 28]. Moreover, in fetuses with zero and reverse (negative) blood flow in the AP, marginal attachment of the umbilical cord to the placenta, a significant degree of obliteration of villous vessels, concentric thickening of their intima and a decrease in the degree of branching of these vessels are often observed, which quantitatively correlates with changes in S/D in the AP [28-30].
Obviously, to understand the pathophysiology of damage to the fetoplacental circulation, a more thorough study of the function of the vascular endothelium is necessary. It is the endothelium that plays a key role in the regulation of vasomotor tone, the balance of the synthesis of pro- and antiangiogenic activity of growth factors, the regulation of inflammatory mediators, and the transport of nutrients and oxygen through the placenta [31, 32]. At the end of normal pregnancy, the total length of the placental microvascular endothelium reaches 550 km, and the total area through which nutrients are transferred from mother to fetus is 15 m2 [33].
Despite the fact that the endothelial cells of the umbilical cord artery and vein, and placental villi belong to the same organ, they all demonstrate significant functional heterogeneity. Thus, lateral mechanical deformation of a cell culture obtained from the endothelium of the AP causes significantly greater synthesis of the vasoconstrictor endothelin-1 than a similar deformation of a culture of umbilical vein endothelial cells [34]. Probably, this feature allows the vein to maintain sufficient internal lumen within a wide range of changes in blood flow through it.
The majority of endothelial cells belonging to the vessels of various organs show the presence of estrogen receptors [35]. Depending on the specific properties of these receptors and the tissues in which they are present, the response of these receptors to increased estrogen concentrations can lead to the expression of the synthesis of proteins that have both vasoconstrictor and vasodilating effects [36–38]. During normal pregnancy, the expression of genes regulating the synthesis of estrogen β-receptors, associated primarily with the vasoconstrictor effect, is more pronounced in endothelial cells of the AP than in vein cells [39, 40]. Moreover, the expression level of these genes in fetuses with FGR and negative diastolic blood flow in the AP was higher than in fetuses with FGR without such hemodynamic disorders [41].
Studies of villous vascular endothelial cells have shown significant heterogeneity between arterial and venous cells. Predominant expression of genes associated with lipid transport activity and metabolism was observed in venous endothelial cells [42]. In contrast, arterial endothelial cells showed greater expression of genes associated with the synthesis of the vascular endothelial growth factor (VEGF) family [42]. The general biological role of these factors is to stimulate angiogenesis and stabilize already formed vessels [43–45]. This proves the leading role of arterial endothelial cells in the formation of the vascular network of the fetus and placenta.
Placental vascular endothelial gene expression is under significant epigenetic influence. A study of the degree of gene methylation showed that venous endothelial cells of the chorionic plate show a higher degree of hypomethylation than arterial endothelial cells [46]. Thus, the proximal promoter region of the endothelial NO synthase gene in venous endothelial cells was methylated to a significantly lesser extent than the similar region in arterial endothelial cells [46, 47]. This, in turn, was expressed in a relatively high level of synthesis of the vasodilator nitric oxide by venous cells. Placentas of fetuses with FGR show a decrease in methylation in the same promoter region of arterial endothelial cells, indicating a likely compensatory increase in NO synthesis in the arterial segment of the placenta [48].
In most vascular systems of the body, the lumen of arterioles to the greatest extent determines the level of peripheral resistance, which is regulated by the activity of the autonomic nervous system and humoral influences [49]. However, the vessels of the chorionic plate and villi are characterized by a lack of innervation [50]. Their tone is completely determined by the local production of vasoactive mediators, most of which are synthesized by endothelial cells [51, 52]. The blood vessels of the placenta also react in a unique way to other vasoactive factors. For example, only placental vessels respond by contraction, not relaxation, to prostaglandin E2 [53]. A decrease in the sensitivity of smooth muscle cells of the walls of these vessels to such vasoactive mediators as acetylcholine, bradykinin and angiotensin II has been shown [54, 55]. NO and endothelin-1 synthesized by the endothelium play an important role in the regulation of vascular tone of the placenta [50]. A significant increase in the concentration of endothelin-1 in the blood of fetuses with FGR, obtained during diagnostic cordocentesis, and a simultaneous decrease in the concentration of vasodilators (6-ketoprostaglandin F1) were shown [56]. Simultaneously with a decrease in NO synthesis, a decrease in the transport of the precursor of this compound, L-arginine, in the endothelial cells of the umbilical cord vein of fetuses with FGR has been shown [57].
In addition to changes in the synthesis of mediators during placental insufficiency, modulation of the electrical properties of endothelial cells has been shown as a result of a decrease in the expression of genes responsible for the synthesis of potassium channels in endothelial cell membranes. In turn, a decrease in the potassium permeability of the membranes of these cells is associated with an increase in the basal tone of the arteries and veins of the chorionic plate [58, 59].
Angiogenesis of fetoplacental vessels
Along with the humoral regulation of vasomotor tone, the anatomical configuration of the villous vessels is of great importance for maintaining effective perfusion of the placenta. Vasculogenesis (formation of new vessels) normally begins in the placenta at 6 weeks and leads to the formation of tertiary villi [60]. As pregnancy progresses, these villi continue to differentiate and transform into immature intermediate villi and then into stem villi. At the same time, angiogenesis (formation and growth of existing vessels) gradually accelerates. At the same time, at approximately 25 weeks, the rate of angiogenesis increases significantly, providing an exponential increase in the length of the villus capillaries [33, 61]. Such a rapid increase in the vascular network of the placenta is necessary for the normal delivery of oxygen and nutrients to the fetus and is reflected in a progressive decrease in S/D in the AP. Morphological studies of placentas with pronounced FGR, zero and negative diastolic blood flows demonstrated a decrease in the degree of branching of the villi capillaries, and their diameter was smaller than in the normal development of pregnancy [62, 63]. At the same time, the density of the capillary network of the villi was also reduced in FGR [64].
The mechanism of such vascular changes is not fully understood, but it is clear that endothelial dysfunction plays an important role in the process of impaired angiogenesis. This disorder is accompanied by an increase in the permeability of the vascular walls and partial degradation of the basement membrane of endothelial cells [65].
As FGRP develops, an imbalance in the synthesis of pro- and antiangiogenic growth factors is observed. The synthesis of VEGF, soluble fms-like tyrosine kinase 1 (sFLT1), placental growth factor (PlGF) and fibroblast growth factor type 2 (FGF2) changes. All these factors are necessary for normal growth of the placental vascular system [66, 67]. A comparative description of their participation in the occurrence of FGR indicates that, probably, the leading role in the disruption of the formation of the fetoplacental vascular network is played by a disruption in the synthesis of factors of the VEGF family [68, 69]. An imbalance in the concentrations of pro- and antiangiogenic factors in FGR has been established both in the blood of the fetus and in the blood of the mother [70–72]. At the same time, a positive correlation was shown between the value of the pulsatility index in the AP, reflecting the peripheral resistance of the placental vessels, and the concentration of the antiangiogenic factor sFLT1 [70]. The proangiogenic factor PlGF, on the contrary, shows a negative correlation with the pulsatility index [67].
Conclusion
Studies of the mechanisms that control fetal growth show that not only maternal nutrition and the state of her blood circulation, but the growth and development of the fetoplacental vascular system are important determinants of the dynamics of fetal weight gain. The study of ultrasound characteristics of blood flow in various vascular segments of the placenta, fetus, mother and comparison of these parameters with the morphological picture and the results of assessing the concentrations of pro- and antiangiogenic growth factors made it possible to obtain important information of both theoretical and practical importance. The vascular network of the placenta is a unique transport system, completely devoid of innervation and regulated exclusively by humoral factors, the main source of which are endothelial cells. During pregnancy complicated by FGR, the imbalance in the synthesis of these compounds shifts in favor of vasoconstrictors and antiangiogenic factors.
Due to the widespread use of assisted reproductive technologies (ART), among which the leading ones are in vitro fertilization methods, studies have appeared showing that fetuses conceived using these technologies have an imbalance in the synthesis of pro- and antiangiogenic vascular growth factors [71, 72] . In addition, early hemodynamic changes characteristic of an increase in peripheral vascular resistance of the placenta were noted in embryos and fetuses conceived using ART [71–74]. In children born using ART, arterial and pulmonary hypertension, intimal thickening of arterial vessels, and cardiac remodeling are observed in a large percentage of cases [73, 74]. All these facts suggest that conception through ART may also induce endothelial dysfunction. Thus, studies of this function, the synthesis of vascular growth factors and assessment of hemodynamics are important not only for improving perinatal outcomes in fetuses with FGR, but also for preventing the programming of cardiovascular diseases in children born using assisted reproductive technologies.
The authors declare no conflict of interest.
Consequences of violations identified during the inspection
Timely diagnosis detects the emergence and development of pathological conditions at the earliest stages. The accuracy of examinations largely depends on the doctor’s experience and the availability of modern equipment.
When referring for Dopplerography of uteroplacental blood flow, the procedure must be completed without fail. Otherwise, negative consequences are possible:
- abnormalities in child development;
- fetal death;
- increased likelihood of miscarriage;
- low birth weight of the child;
- hormonal imbalance;
- pathologies of the heart and blood vessels.
The good health of the unborn child is the most important incentive for a happy life for most people. Correct implementation of DPM can detect the presence of developmental disorders in the early stages - and respond in a timely manner.
The Harmony clinic has everything you need to carry out Doppler measurements: qualified doctors, modern equipment, affordable prices and first-class service. If you want the procedure to be guaranteed to be not only accurate, but also safe for the baby, then come to us!