VSD. Who came across it?
@Mom_since_this_year Hello everyone! I want to share our story! My pregnancy went well, a couple of times during pregnancy I had a mild cold, I didn’t take any serious medications, everything went away on its own. The ultrasound did not reveal any abnormalities in the fetus. The birth was natural and went well. The nerves started on the second day after the birth of our son. During the rounds, the doctor heard a noise, but then he immediately reassured me that this happens, he will listen again tomorrow. The next day the noise intensified, the baby was scheduled for a heart ultrasound. And then everything was like in a dream, a cardiologist came and announced the diagnosis: congenital heart disease - perimembranous ventricular septal defect 4.5 mm. Tears, fear, reading all the forums, articles that say that these defects do not heal on their own. Four days later we are transferred to the Bashlyaeva Children's Hospital, where we spend three terrible weeks, a specialist from Bakulevka comes there on Wednesdays and does an expert ultrasound, the numbers on the defect that are announced there make me completely depressed - 8.5 mm. There the child is given medications Verashpiron and Digoxin. Three weeks later, they measure a 7.2 mm defect and send us home to register with a cardiologist at the clinic. Also at the hospital we were told that open heart surgery was inevitable before the year was over. While still in the hospital, I made an appointment with cardiologists at the Morozov and Filatov hospitals. At home they continued to give all the medications. First, we arrived for an appointment at the Morozov hospital, where they measured a 5 mm defect on an ultrasound and said that with it there was a chance to wait for surgery in 5 years through an occluder, we exhaled a little. We made an appointment with V.A. Kryukov at the Filatov hospital. he measured 4.5 mm and said that from his experience he thought that our defect should close. Next we made an appointment with M.M. Belyaeva. also at the Filatov hospital, she also measured 4.5 mm, but was not so optimistic in her forecasts. We decided to register with her. And then, first every month, then every two, three months, we went for an ultrasound and each time the defect became smaller. The child was gaining weight well, was active, and did not at all look like he had congenital heart disease. So at 9 months we again went for another ultrasound, which was performed by Yu.Yu. Kornoukhov. and about happiness, the words that all parents dream of hearing, your child is HEALTHY!!! Imagine, HEALTHY! Our defect lasted for 9 months. Everyone congratulated us and sent us home in peace. At the same time, the doctor said that many parents, having heard the same or similar diagnosis, rush to have surgery, but very often such defects are closed and we confirm this! Conclusion: consult several doctors from different clinics, do not rush to make serious decisions if time permits, we were also told that good weight gain in a child has a positive effect on healing. We are 9 months old and weigh 11 kg. And of course, Faith and prayers to God. May all children be healthy.
Isolated ventricular septal defect (VSD)
Ventricular septal defect is the most common congenital heart defect. Defect, i.e. a hole in the septum separating the right and left ventricles is the only violation of the normal development of the heart, and then they speak of an isolated defect or part of another, more complex defect, for example, tetralogy of Fallot. In this section we will only discuss isolated defects.
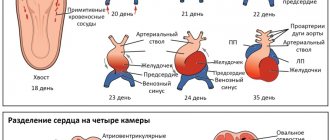
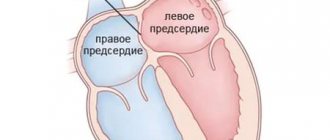
The interventricular septum is a powerful muscular barrier that forms the internal walls of both the right and left ventricles, and in each it makes up approximately 1/3 of their total area. It is also involved in the process of contraction and relaxation of the heart during each cycle, like the rest of the ventricular walls. In the fetus it is formed from three components. At the 4-5th week of pregnancy, all these components must be accurately matched and connected to each other, as in a Lego constructor. If for some reason this does not happen, a hole or defect remains in the septum. Soon after birth
and the establishment of normal blood flow in both circulations, a significant difference in pressure arises between the left and right ventricles. And then the blood from the left ventricle begins to be pumped simultaneously into the aorta, i.e. where it should be, and through the defect - into the right ventricle, where it should not be. Those. With each systole, a reset occurs from left to right. In such a situation, the right ventricle is forced to work with increased load in order to pump this excess volume, and what’s more, already oxidized blood, back to the lungs and to the left sections.
The amount of discharge depends on the size and position of the defect: it can be small
and have almost no effect on the work of the heart, but can be huge, with a diameter as large as the mouth of the aorta.
Types of ventricular septal defects
A few words about the types of defects. They may be “typical”, i.e. the most common, and occupy the area of the upper part of the septum. They can be muscular, i.e. located closer to the apex, and finally high, under the pulmonary valves, single or multiple (i.e. more than one).
Let's consider the most common and “good” option. The child was born of normal weight, full-term and, of course, very beautiful. But they already told you in the maternity hospital that he has a “heart murmur.” If there or somewhere nearby there is an echocardiography machine and a pediatric cardiologist, it is imperative to do a study to understand what this noise is caused by, whether it is important or not, whether it will affect it in the future .
But let’s assume that the defect was seen on an ultrasound and the diagnosis was made.
The child, however, looks normal, eats well and is gaining weight, is not sick with anything and, in fact, apart from a heart murmur, nothing upsets either you or him. Remember: the louder the noise, the smaller the defect. This is a situation to which the title of W. Shakespeare’s famous play “Much Ado About Nothing” fits perfectly. The release that this noise gives can sometimes even be felt if you place your palm on the left side of the child's chest. But in order to calm down, you need to make sure that the noise is due only to a small defect
of the interventricular septum and nothing else. And for this you need to be observed by a pediatric cardiologist, and an electrocardiogram and ultrasound are taken every three months.
In most cases, approximately 65-75%, such defects close on their own, spontaneously, and if additional symptoms do not appear, you can safely wait 4-5 or even more years. But, if the child has reached school age and remains asymptomatic, then you may nevertheless be offered surgery. The fact is that if a child falls ill with any childhood infection or even with the simple removal of a damaged tooth in the presence of a ventricular septal defect, the development of endocarditis is possible, i.e. inflammatory process of the inner lining of the heart chambers. And, although this probability is very small - only 1-2% of cases, it exists. In this case, the defect is closed for preventive rather than clinical reasons, and no one has proven that this should not be done.
However, before you agree to the operation, in this case, please, without offending anyone
, try to find out what experience is available in the medical institution where you went, what are the results of operations there, what is the degree of risk.
Since the child’s condition is almost normal, and there is always a risk of any intervention, we must calmly weigh everything. No, we are far from urging you to refuse surgery. We just want to advise you to be very thorough, because no one will ever give you written guarantees: an operation is an operation, even if it is performed in an x-ray surgery room (as they will most likely offer you) and complications are always possible, and you will sign a document confirming your agreement. And the final decision is your responsibility.
And one more thing - never suggest to such a child that he has
a bad heart.
Do not protect him from the physical activities available to him,
do not make him a “disabled person.”
Excessive “protection” and prohibitions can lead to the most unfavorable consequences in the formation of his character.
But this is a separate topic, and it concerns not only VSD.
Large defects are another story, much more dangerous. Immediately after the baby's first breath, the blood flow from the left ventricle is divided into two - into the aorta and into the defect, and they are equal in volume! Not only the heart, but also the blood vessels of the lungs find themselves in a difficult situation: the right sections and vessels of the lungs are filled with an increased volume of excess blood entering through the defect. The most important indicators of this development of events are the pressure in the pulmonary artery and the amount of discharge. These data are provided today by ultrasound and, of course, probing of the cavities of the heart. An increase in pressure in the pulmonary circle indicates pulmonary hypertension - the most dangerous consequence of a large discharge from the left to the right. Outwardly everything is more or less working out. Numerous compensation mechanisms are activated: the muscle mass of the ventricles increases, the vessels of the lungs also adapt, first taking in excess blood volume, then thickening the walls of the arteries and arterioles, making them denser and less elastic. This period is dangerous, because... the child's condition may clinically improve significantly, but this improvement is deceptive, and the moment for surgical intervention may be missed. If this situation continues for quite a long time - several months or years, then at some point the pressures in the right and left ventricles are compared in all phases of the cardiac cycle and discharge through the defect no longer occurs
.
And then the pressure in the right ventricle may turn out to be higher
than in the left, and then the so-called “
reverse discharge
” begins, or venous blood will flow through the defect into the arterial system - into the systemic circle.
The patient turns blue.
We painted this picture to make it clear that a defect such as a ventricular septal defect, which is very easy and safe to close in the
early stages
, becomes a defect in which closure loses its meaning, and it is too late to operate.
We are talking here, let us remind you, only about large defects
or about those cases when there are several holes in the partition. Fortunately, this happens much less frequently than most cases.
What should you pay attention to in order to avoid such developments in time?
All of the above applies only to ventricular septal defects, which occur absolutely without complaints, in completely healthy (otherwise) children without any other signs of heart failure. What should you pay attention to with this defect?
The main indicator of the newborn period is weight gain. Let's say an infant eats poorly and therefore gains little weight. His appetite is normal, but due to shortness of breath it is difficult for him to suck, but for now this is his main and rather difficult job. He cries a lot because he can never get enough. In older children, against this background, frequent colds occur, which become prolonged and can develop into pneumonia. This may continue for several months, and if the cause is a VSD, such a child should be under constant supervision of a cardiologist, and if the symptoms do not go away, he will probably receive heart drugs in the form of digoxin or digitalis - the good old drug that improves heart function, and maybe even mild diuretics. He now has signs of heart failure, which can be treated conservatively. But only for now.
When should such a large defect be closed?
With drug therapy, symptoms may go away or significantly decrease. But if nothing changes, if the size of the heart increases and the size of the defect on ultrasound remains the same, you need to contact surgeons.
In the first few months of life, ventricular septal defects, even large ones, may shrink or close on their own. If the child does not get better, you cannot wait, because the situation may turn into the one we described at the beginning, and it will be too late to operate.
The best results
Surgeries occur after the removal of large VSDs at the age of
two to two and a half years
, when the child has signs of heart failure. Then all processes are still reversible. The heart quickly realizes that it is now much easier than before. It quickly decreases in size and blood flow in both circles normalizes.
After the operation, the child is practically healthy and there is no reason to classify him as a member of the group of so-called “disabled children”, as is sometimes done in medical institutions. He can do anything
, and will quickly forget about what happened to him, and except for the scar on his chest, nothing will remind him of it.
So, you are offered an operation, which is now necessary.
and absolutely shown. Only she will heal the child and remove from you the constant feeling of a threat to his life. The operating surgeon will tell you what your case is about and what he or she plans to do. Technical difficulties (for example, the need to close not one, but several defects) can make the operation more complex and lengthy. In an unusual, rare situation, the surgeon will definitely explain everything to you, and your task is to try to understand everything that they tell you, ask the right questions and calm down.
The operation to eliminate a ventricular septal defect is an open
, since it is necessary to open the cavities of the heart, and therefore it is done using artificial circulation.
Ventricular septal defects are closed by suturing the hole (i.e., simply placing several stitches) or, most often, using a patch made of synthetic (or specially treated biological) material, which is quickly covered with the heart’s own tissue.
Nowadays, X-ray surgical methods for closing defects are also used, but this is not always possible; it depends on the anatomical location of the defect, and also on the qualifications of the X-ray surgeon. Traditional surgery for ventricular septal defects is one of the most common and well-established congenital heart defects in surgery, and its results are excellent. So if there is evidence, you don’t need to doubt it.
Radical surgery cannot always be done. In case of a very large defect in a child of too little weight, malnourished, with signs of heart failure that cannot be treated conservatively, the very factor of a major operation with artificial circulation can be dangerous, especially when it comes to children in the first months of life
. And then there is a way out: to break the surgical treatment into two stages - first to help the heart improve its condition, and then to finally close the defect.
It is possible to reduce shunt from the left ventricle to the right by increasing the resistance to right ventricular ejection. By this we will achieve a decrease in pressure in the pulmonary artery system, firstly, below the artificially created obstacle to blood flow, we will equalize the pressure in the right and left ventricles above it (obstacle) and, as a result, the volume of the discharge itself decreases. And this is achieved simply: a cuff is placed on the pulmonary artery above its valves, which will narrow the artery to approximately ½ of the lumen.
An artificially created obstruction to blood flow gives us the desired hemodynamic result. The second operation is performed after a few months, no more. In no case should you wait several years, although the child’s condition may no longer inspire any concern.
However, the postoperative period after surgery to narrow the pulmonary artery is very difficult; children have a hard time with this auxiliary intervention, and that is why we are now striving for a one-stage radical correction of the defect. Even severe children recover from heart failure faster after radical correction of the defect; all life-threatening symptoms gradually disappear.
The operation of narrowing the pulmonary artery is “auxiliary”. It should be in service where there are no conditions for the safe carrying out and nursing of radical corrections to children
.
The final stage of treatment consists of removing this cuff
and
closing the defect
. It is already done under conditions of artificial circulation and is not associated with much risk, especially when a relatively short period of time has passed between it and the first stage.
Summarize. VSD is a common congenital defect that may be accompanied by the early onset of heart failure and the subsequent development of irreversible pulmonary hypertension.
Surgical treatment is the only method and allows you to completely eliminate the defect and its consequences. It must be timely, and the operation itself must be safe. The operation can be elective or urgent, but almost never emergency. In some cases, it can be divided into two stages with an interval of 6-12 months. The appearance of signs of “reverse discharge” in a child with an isolated VSD is a sign of lost time. And with all modern examination methods, such a complication cannot be justified in any way, and part of the blame will fall on you, since you also did not notice or did not want to notice that the moment was missed.
How to get treatment at the Scientific Center named after. A.N. Bakuleva?
Online consultations
Treatment of the defect
Treatment is not required in two cases: if the defect consists of muscle tissue (regardless of its size and location, it can be expected to close on its own), and also if the hole is small and the child is no more than two years old. Small injuries can heal in early childhood, so only observation is required.
The remaining patients require a specific operation - closure of the ventricular septal defect. Despite the fact that they try to carry out the procedure as early as possible, sometimes it is necessary to wait some time. In this case, maintenance drug therapy is prescribed to reduce the load on the heart muscle and support blood vessels. There are two fundamentally different approaches to surgery: endovascular treatment and open surgery. The latter option is technically complex - special equipment is required to maintain artificial blood circulation, and the rehabilitation period can be fraught with complications.
Congenital heart defects in children with Down syndrome
It is well known that congenital cardiac anomalies occur in almost half of children with Down syndrome and have a major impact on infant survival. Since the middle of the last century, many studies have been conducted to identify the frequency, specificity and nature of heart defects in these children. So, in the period 1970–1980s. There was an increase in the prevalence of congenital anomalies of the cardiovascular system in patients with Down syndrome. This was mainly due to improved diagnosis of patent ductus arteriosus and atrial septal defect (MJ Khoury, JD Erickson, 1992). According to foreign authors, the most common cases of Down syndrome are ventricular septal defect, atrial septal defect, common open atrioventricular canal, tetralogy of Fallot and other defects, accounting for less than 1%.
Over the years of scientific observations, it has become obvious that to identify congenital heart disease in a newborn with Down syndrome, a physical examination, including examination and auscultation, is mandatory, but not sufficient. Thus, McElhinney et al. found that the information content of a physical examination for identifying cardiac abnormalities in children with Down syndrome does not exceed 80%. It turned out that 15 of the 114 children studied had no signs of congenital heart defects upon examination, but upon ultrasound examination they were diagnosed with cardiac anomalies, and nine of them subsequently required surgical treatment.
Materials and methods
We conducted a study of the incidence and characteristics of the clinical picture of congenital heart defects and persistent fetal communications in 522 children with Down syndrome aged 0 to 8 years, raised at home. The children received medical, psychological and pedagogical assistance at the Early Help Center of the Downside Up Charitable Foundation, where they were observed from the moment of application (age at the first visit varied from 0 to 7 years) until 8 years. At the initial visit, anamnesis was collected, a clinical examination, and medical documentation were analyzed. All children, even in the absence of clinically significant symptoms of heart disease, were sent for electrocardiographic and echocardiographic examinations and, if necessary, for treatment in the appropriate specialized cardiology hospitals and dispensaries.
results
In all patients, Down syndrome was confirmed by chromosomal testing. Regular trisomy of the 21st chromosome was detected in 499 children (499/522), which was 90.4%, translocation form - in 24 (4.3%), mosaicism - in 28 (5.1%), in one child trisomy of the 21st and X chromosomes (karyotype 48,ХХХ,+21) – 0.2%.
The results of an echocardiographic study of 428 children were obtained (see figure). Congenital heart defects were diagnosed in 195 (195/428), which amounted to 45.6 % . In the structure of these anomalies, children with Down syndrome often had an atrial septal defect (ASD), namely in 30.2% (59/195) of cases. Common atrioventricular canal (CAVC) was 24.1% (47/195), ventricular septal defect (VSD) – 23.1% (45/195), combination of atrial and interventricular septal defects (ASD+VSD) – 10.8% . Other defects, such as tetralogy of Fallot, pulmonary stenosis, etc., totaled 11.8% (23/195). Persistence of a hemodynamically significant patent ductus arteriosus (PDA) requiring surgical intervention was detected in 2.8% (12/428).
Structure of cardiac abnormalities in children with Down syndrome
Almost all of the children we studied were born full-term. The term of delivery was 38.2 ± 1.3 weeks. However, when assessing the anthropometric data of newborns with Down syndrome and congenital heart defects, it turned out that their physical development suffers even in utero. Physical development delay (IUGR) - birth weight below the 10th percentile in accordance with gestational age in comparison with the indicators of physical development of G. M. Dementieva, E. V. Korotkaya - was noted in 18.7% of children. All newborns with cardiac anomalies had an asymmetric form of IUGR (Pounderal Index, PI>25). Probably, the delay in physical development was formed under the influence of, mainly, a non-genetic factor.
It is known that newborns with Down syndrome often experience morphofunctional immaturity (according to our data, it occurs in 17.9% of cases). In children with morphofunctional immaturity, the size of the atrial septal defect, which is considered as an open foramen ovale even with its hemodynamic significance, and the ductus arteriosus are often underestimated, while circulatory failure occurs. Congestive pulmonary hypertension leads to the development of pneumonia. The occurrence and prolonged course of pneumonia in children with Down syndrome is explained by their characteristic immunological disorders.
It is well known that the manifestation of heart failure in young children, unlike in older children, can occur under the guise of other conditions. In addition to classic symptoms such as tachycardia (increased heart rate), tachypnea (increased respiratory rate), cyanosis of the skin and mucous membranes, sluggish sucking and decreased rates of physical and psychomotor development are typical. In such cases, pediatricians have certain difficulties in carrying out differential diagnosis if a child has Down syndrome. In such children, clinical symptoms of circulatory failure can be regarded as manifestations of psychomotor development characteristics typical of Down syndrome. So, if feeding difficulties arise: the child is lethargic, reluctant to take the breast or pacifier, sucks sluggishly, cannot suck out the required amount of nutrition, up to a complete refusal to feed, such problems are often explained by muscle hypotonia, general lethargy, characteristic of children with Down syndrome, followed by the appointment of a restorative massage, which worsens the child’s condition. Subsequently, poor weight gain is noted. She directs clinicians to identify pathologies of the gastrointestinal tract, hypogalactia in the mother, and study the quality of milk and its contamination. In the fight against progressive malnutrition, the baby is often transferred to artificial formula. It is important to note that malnutrition may cause a delay in surgical treatment of heart disease and/or adversely affect its outcome.
Thus, the weak focus of pediatricians on identifying symptoms of circulatory failure in a child with Down syndrome makes it difficult to diagnose it in a timely manner, and, consequently, to adequately treat congenital heart disease.
A clear example would be the analysis of a medical history.
Andrey B., from the first, physiologically proceeding pregnancy. Delivery on time. The boy’s weight at birth is 3000 g, height is 51 cm, APGAR score is 88b. The child's condition after birth is satisfactory. Signs of morphofunctional immaturity and phenotypic signs of Down syndrome were noted. In order to confirm chromosomal pathology, blood was taken to determine the karyotype. Regular trisomy of chromosome 21 was revealed. From the first day of life, a systolic murmur was noted during auscultation of the chest. To exclude cardiac developmental anomalies, an echocardiogram was performed and a patent foramen ovale measuring 4 mm was detected. The child was discharged home under the supervision of a local pediatrician and cardiologist at his place of residence. Subsequently, the boy became lethargic, reluctant to take the breast, frequent regurgitation and rare bowel movements were noted. Over the course of a month, the child gained 210 g in weight. During examination, signs of circulatory failure were noticed: shortness of breath at rest, moderate tachycardia. The boy was sent to the Scientific Center for Agricultural Surgery named after. Bakulev, where a heart defect was diagnosed - an atrial septal defect measuring 6 mm with a significant impairment of cardiac hemodynamics. A chest x-ray showed expansion of the roots of the lungs, CTI = 57%. According to the ECG: deviation of the electrical axis of the heart to the right. At the age of 4 months, surgical treatment of atrial septal defect was performed.
Advances in cardiac surgery over recent decades have increased the survival rate of infants with Down syndrome and cardiovascular disease from 78% in 1985 to 90% by 2004 (Claire Irving et al., 2008).
Hijii T. et al. (1997) reported that 87.8% of patients with Down syndrome who underwent surgical treatment of congenital heart disease survive to the age of 24 years.
When comparing the course and outcomes of surgical treatment of the complete form of the atrioventricular canal in infants with Down syndrome and without the syndrome, in work carried out on the basis of the Scientific Center for Surgery named after. Bakuleva, T.I. Zadko notes that children with Down syndrome develop faster pulmonary hypertension, an important mechanism in the development of which, obviously, is oxidative stress. Genetically determined features of the antioxidant system, including initially low levels of glutathione and higher antioxidant activity of serum in children with Down syndrome (N.P. Kotlukova, O.I. Artemenko et al., 2008), indicate higher oxidative stress in development of pulmonary hypertension with heart defects with pulmonary hypervolemia.
Of the early complications of surgical correction of the atrioventricular canal, infectious and septic complications are more common in children with Down syndrome, while acute heart failure is most common in children without the syndrome (T.I. Zadko, 2005). This circumstance is explained by the anatomical features of the defect and the existing immunological disorders in infants with trisomy of the 21st chromosome.
conclusions
Our data on the frequency of cardiac anomalies do not contradict those already known in the literature. About half of children with Down syndrome have pathology of the cardiovascular system: 45.5% have congenital heart defects, 2.8% have a hemodynamically significant patent ductus arteriosus.
Analysis of the data from the studies, as well as our own results, makes clear the need for early cardiac examination of all newborns with Down syndrome, including, in addition to examination and auscultation, echocardiological and electrocardiological studies. A careful approach and assessment of clinical symptoms, as well as knowledge of the genetically determined characteristics of children with Down syndrome will help to timely diagnose circulatory failure and begin adequate therapy. All children with identified heart defects should be consulted by a cardiac surgeon to determine the need and timing of surgical treatment.
Literature
- Zadko T.I. Down syndrome in combination with the full form of atrioventricular communication: relevance, diagnosis, concomitant pathology, anatomy, features of the natural history, results of surgical treatment // Children's diseases of the heart and blood vessels. – 2005. – No. 6. – P. 10–18.
- The role of oxidative stress and the antioxidant system in the pathogenesis of congenital heart defects / N. P. Kotlukova, O. I. Artemenko, M. P. Davydova, O. N. Ilyina, L. A. Kurbatova // Pediatrics. – 2009. – T. 87, No. 1. – P. 24–28.
- Cassidy SB, Allanson JE Management of Genetic Syndromes. 2nd ed. – P. 191–210. URL: https://www.wiley.com/en-us/Management+of+Genetic+Syndromes%2C+3rd+Edition-p-9780470191415
- Correlation between abnormal cardiac physical examination and echocardiographic findings in neonates with Down syndrome / DB McElhinney, M. Straka, E. Goldmuntz, EH Zackai // American Journal of Medical Genetics. – 2002. – Part A. – P. 238–241.
- Khory MJ, Erickson JD Improved ascertainment of cardiovascular malformation in infants with Down syndrome, Atlanta, 1968 through 1989 // Epidemiology. – 1992. – Vol. 136. – P. 1457–1464.
- Life expectancy and social adaptation in individuals with Down syndrome and without surgery for congenital heart disease / T. Hijii, J. Fukushige, H. Igarashi et al. // Clinical Pediatrics. – 1997. –Vol. 36. – P. 327–332.
- Twenty-year trends in prevalence and survival of Down syndrome / C. Irving, A. Basu, S. Richmond et al. // European Journal of Human Genetics. – 2008. – Vol. 16. – P. 1336–1340.
Benefits of endovascular surgery
This is a relatively young method of treatment - endovascular heart surgery became possible in the 21st century. Endovascular is a treatment that does not require abdominal surgery. The operation is performed using a special device called an occluder.
Endovascular treatment is suitable for the majority of patients with ventricular septal defect. The operation can be performed on children if they have reached a weight of 5 kg - those who are smaller have insufficient vascular lumen. If the patient’s condition allows, it is better to carry out the procedure as quickly as possible. For children, the recommended interval is from 1 to 3 years.
To carry out manipulations, an X-ray operating room is required - this is a sterile room equipped with special equipment to control the surgeon’s manipulations. An occluder is a device that opens up and acts as a patch. It is placed in a catheter, which is passed to the heart through a large vessel. This way, no cutting or damage is required. One puncture is enough at the catheter insertion site - usually the thigh or upper limb. A correctly installed occluder blocks the pathological discharge of blood from one ventricle to another. If it is installed incorrectly or moves after surgery, it is enough to repeat all the manipulations again and install it in the desired position.
The duration of the procedure is no more than 2-3 hours. Long-term hospitalization is not required - observation for 24 hours in the intensive care unit is sufficient. After another 24 hours, the patient is discharged from the hospital. After this, regular consultations with a cardiologist and monitoring and diagnostic measures are required to ensure that the occluder does not move. Complications occur extremely rarely, most occur in the first day. Within six months, the occluder is covered with heart cells and completely takes root. After this, the risks are minimal.
Publications in the media
Ventricular septal defect (VSD) is a congenital heart defect with communication between the right and left ventricles.
Etiology • Congenital malformations (isolated VSD, part of a combined congenital heart disease, for example, tetralogy of Fallot, transposition of the great vessels, truncus arteriosus, tricuspid valve atresia, etc.) • There is evidence of autosomal dominant and recessive types of inheritance. In 3.3% of cases, this defect is also found in direct relatives of patients with VSD • Rupture of the interventricular septum due to trauma and MI.
Statistical data • VSD accounts for 9–25% of all congenital heart defects • Is detected in 15.7% of live-born children with congenital heart disease • As a complication of transmural myocardial infarction - 1–3% • 6% of all VSDs and 25% of VSDs in infants are accompanied by a patent ductus arteriosus, 5 % of all VSDs - coarctation of the aorta, 2% of congenital VSDs - aortic valve stenosis • In 1.7% of cases, the interventricular septum is absent, and this condition is characterized as a single ventricle of the heart • The ratio of male to female sex is 1:1.
Pathogenesis. The degree of functional impairment depends on the amount of blood discharge and total pulmonary vascular resistance (TPVR). When shunting from left to right and the ratio of pulmonary minute volume to systemic blood flow (Qp/Qs) is less than 1.5:1, pulmonary blood flow increases slightly, and no increase in PVVR occurs. With large VSDs (Qp/Qs more than 2:1), pulmonary blood flow and pulmonary blood flow significantly increase, and pressures in the right and left ventricles are equalized. As the blood volume increases, the direction of blood discharge may change - it begins to occur from right to left. Without treatment, right and left ventricular failure and irreversible changes in the pulmonary vessels (Eisenmenger syndrome) develop.
Variants of VSD • Membranous VSD (75%) are located in the upper part of the interventricular septum, under the aortic valve and the septal cusp of the tricuspid valve, often close spontaneously • Muscular VSD (10%) are located in the muscular part of the interventricular septum, at a considerable distance from the valves and conduction system , are multiple, fenestrated and often close spontaneously • Supracrestal (VSD of the right ventricular outflow tract, 5%) are located above the supraventricular crest, often accompanied by aortic regurgitation of the aortic valve, do not close spontaneously • Patent AV canal (10%) is found in the posterior part of the interventricular septum, near the site of attachment of the rings of the mitral and tricuspid valves, often found in Down syndrome, combined with ASD of the ostium primum type and malformations of the leaflets and chords of the mitral and tricuspid valves, does not close spontaneously • Depending on the size of the VSD, small ones are distinguished (Tolochinov-Roger disease ) and large (more than 1 cm or half the diameter of the aortic orifice) defects.
Clinical picture
• Complaints: see Atrial septal defect.
• Objectively • Pallor of the skin • Harrison's furrows • Increased apical impulse, trembling in the area of the left lower edge of the sternum • Pathological splitting of the second tone as a result of prolongation of the ejection period of the right ventricle • Rough pansystolic murmur at the left lower edge of the sternum • With supracrestal VSD - aortic diastolic murmur insufficiency.
Instrumental diagnostics
• ECG: signs of hypertrophy and overload of the left parts, and with pulmonary hypertension - also of the right.
• Jugular venography: high-amplitude A waves (atrial contraction with a rigid right ventricle) and, sometimes, V wave (tricuspid regurgitation).
• EchoCG •• Hypertrophy and dilatation of the left parts, and in case of pulmonary hypertension - also the right ones •• Visualization of VSD in Doppler and B-mode •• Diagnosis of associated anomalies (valvular defects, coarctation of the aorta, etc.) •• Determine systolic pressure in the right ventricle , degree of shunt and Qp/Qs •• Transesophageal echocardiography is performed in adults.
• X-ray of the chest organs •• For small VSDs - a normal x-ray picture •• Bulging of the left ventricular arch, increased pulmonary vascular pattern •• With pulmonary hypertension - bulging of the pulmonary artery arch, expansion and lack of structure of the roots of the lungs with a sharp narrowing of the distal branches and depletion of the pulmonary vascular system drawing.
• Radionuclide ventriculography: see Atrial septal defect.
• Catheterization of the cardiac chambers •• Indicated for suspected pulmonary hypertension, before open-heart surgery and in case of conflicting clinical data •• Calculate Qp/Qs •• Tests with aminophylline and oxygen inhalation are performed to determine the prognosis regarding the reversibility of pulmonary hypertension.
• Left ventriculography, coronary angiography: visualization and quantification of shunt, diagnosis of CAD in the presence of symptoms or before surgery.
Drug treatment. With an asymptomatic course and normal pressure in the pulmonary artery (even with large defects), conservative treatment is possible for up to 3–5 years of life. If there is stagnation in the pulmonary circulation, use peripheral vasodilators (hydralazine or sodium nitroprusside), which reduce the discharge from left to right. For right ventricular failure - diuretics. Before and for 6 months after uncomplicated surgical correction of VSD - prevention of infective endocarditis.
Surgery
Indications • Asymptomatic - if spontaneous closure of the defect does not occur by 3–5 years of life, although better results are achieved with surgical treatment before the age of 1 year • Heart failure or pulmonary hypertension in young children • In adults, the Qp/Qs ratio is 1 .5 or more.
Contraindications: see Atrial septal defect.
Methods of surgical treatment. Palliative intervention - narrowing of the pulmonary trunk with a cuff, is performed when emergency surgery is necessary for children weighing less than 3 kg, with concomitant heart defects and little clinical experience in radical correction of the defect at an early age. In case of a traumatic defect in the area of the membranous part of the interatrial septum, suturing of the defect is possible. In other cases, the defect is repaired with a patch made of autopericardium or synthetic materials. In case of post-infarction VSD, plastic surgery of the defect is performed with simultaneous coronary bypass surgery.
Specific postoperative complications: infective endocarditis, AV block, ventricular arrhythmias, recanalization of VSD, tricuspid valve insufficiency.
Forecast. In 80% of patients with large VSDs, spontaneous closure of the defect occurs within 1 month, in 90% - before the age of 8 years, there are isolated cases of spontaneous closure of VSDs between the ages of 21 and 31 years. With small defects, life expectancy does not change significantly, but the risk of infective endocarditis increases (4%). With a medium-sized VSD, heart failure usually develops in childhood, and severe pulmonary hypertension is rare. Large VSDs without a pressure gradient between the ventricles lead to the development of Eisenmenger syndrome in 10% of cases; most of these patients die in childhood or adolescence. Emergency surgical intervention is required in 35% of children within 3 months after birth, 45% within 1 year. Maternal mortality during pregnancy and childbirth with Eisenmenger syndrome exceeds 50%. With post-infarction VSD, 7% of patients survive 1 year in the absence of surgical treatment. Hospital mortality after narrowing of the pulmonary artery is 7–9%, 5-year survival rate is 80.7%, 10-year survival rate is 70.6%. Mortality during surgical treatment of post-infarction VSD is 15–50%. In-hospital mortality during closure of isolated congenital VSD with low LVVR is 2.5%, with high LVVR - less than 5.6%.
Abbreviations. Qp/Qs is the ratio of the pulmonary minute volume of blood flow to the systemic one. TPVR - total pulmonary vascular resistance.
ICD-10 • Q21.0 VSD
VSD in the fetus
Hi all. Girls, I’ll say right away that the story has a sad ending. Decide for yourself whether to read it or not. This post is a message to every woman who faces various diagnoses and sentences during pregnancy. To avoid this, you need to move on with your life. Not asking what all this is for, but analyzing what it is all for. So, if you want to hear a story about an enlarged TVP, a chorionic villus biopsy, a verdict from geneticists and a termination of pregnancy at 13-14 weeks, then listen up.
I’ll try not to delve into emotions and feelings, it’s so bad, I just want to howl...
Now I’m lying in the maternity hospital (in the gynecological department) and writing this post. I once dreamed that I would write in January-February 2021, when I give birth, that TVP at the first screening is not so scary and everything is fine. But I’m writing six months earlier, which means...
I'm 37. Happy family. Son and daughter. Beautiful talented children. My husband and I adore each other. And so the whole family decided to give birth to a “little happiness.” My husband and I prepared for this baby like never before: vitamins, proper nutrition, sports, tests (I’m not writing about drinking/smoking, this never happened in our lives). In general, we are ready. The children are waiting impatiently. And now, June 5th is a gift of fate. Almost 2 months of euphoria. Joint discussions, plans. July 23 - first blow. Screening in the perinatal center, TVP 5 mm. Fetal VSD. Referral on July 27 to MONIIAG. July 24,25,26 - read, read, read, what, from whom, how. There are many good cases. And the bad ones. Will all the women who have gone through this write about them? I want to share joy, not sorrow. However, we have put aside all negative forecasts. We cannot have chromosomal abnormalities, but we decided to cure the heart, the heart, with our love. On July 27, I went to MONIIAG with excitement, but without panic. Corridors of hopelessness. Corridors of hope. There were about 25 of us that day. They called us for an ultrasound. After that, they looked at the blood test that was done during the screening and were sent into the corridor to wait. Then each woman was called and the geneticist explained what they didn’t like. Almost everyone was offered a chorionic villus biopsy/amniocentesis/cordocentesis. In the same day. Almost the entire crowd moved gradually from room 10 to room 5. Almost everyone agreed. While they were sitting, they talked a little. All different girls. From 20 to 45. Some cried, some thought, some were in a hurry. Everyone has their own story. But there is one hope. I tried to cheer everyone up. Everyone was sitting and looking for only one thing on the Internet: screening, ultrasound, TVP, biopsy, error, consequences, etc... Then I decided that I would write when everything was fine, in order to support the girls in such situations. Did not work out. There is nothing scary about the biopsy procedure. Exactly physically. The whole procedure takes a minute. I didn’t feel the injection, but when they took in the liquid, it was as if something big had been taken out of me. Such an unpleasant feeling. But very quickly. Then we sat for 10 minutes in the corridor, feeling a little weak. I went to work. It was Monday. I didn’t even think about the call from the geneticists, I was sure that everything was fine. Tuesday, Wednesday, Thursday are regular work and family days. On Friday, July 31, I went to give urine before a scheduled visit to the residential complex (appointment for August 3). I ran to my wife and said that they didn’t call, but I know that everything is fine. I went to work and went to the store. Call. “You're worried about genetics. Unfortunately, the baby has an extra chromosome 13. You need to come on Monday, August 3rd for the conclusion. All. Automatically. I went home and told my husband. I don't write emotions. We just died. I had to go to work. Having forgotten about all my job responsibilities, the first thing I did at work was go online. 13th chromosome. Patau syndrome. One of the most terrible. No, not with me, not with us. A child is born in pain and dies in pain. And not a single positive story. That day, when I picked up my daughter from kindergarten, she ran out to me, I grabbed her and started crying. Crying with happiness that I had two healthy children, that I have my family. I cried with happiness. And I cried from grief, from hopelessness that we were not given a third happiness... If only this concerned only me. And here, the baby, who is doomed and, of course, my family, whose happiness and well-being I cannot risk like that... I tried to hold on during the weekend. With family at home, any pain is dulled. And so on August 3, I’m going to MONIIAG. They give a conclusion and consent to interruption. I'm still hoping they'll tell me it's a mistake. I put my signature, I don’t see where. The eyes cannot see from tears. How? How could this happen? Accident, genetic accident. Incorrect division of the egg and that’s all... Genetics signed the verdict for me, and I – for our baby. To the boy-Vasilka... Further to his native Voskresensk in the residential complex, direction to the hospital. Everything is in the fog. I came to the hospital at 16.00, gave all the documents, and asked if I had a certificate for Covid (smear and antibodies). Without them, it turns out, they won’t accept you. The doctor on duty came and said that there was an emergency here, without these certificates, come tomorrow with your things at 8.00. On August 4 at 8.00 I am in the emergency department. They take documents and ask for certificates for Covid. I explain that yesterday they told me to come without them. But today you can’t do that. Like this. I go to a paid laboratory and take two tests for 4,500. Accepted. Everything is long. At 11.00 I am in the room. One. Doomed. At 12.00 doctor's examination. Term 13-14. Can't clean it. I sign an agreement on medication interruption. The first tablet is given at 12.30. Pack of 3 pieces. Drink every other day. The doctor strictly gives it to me and watches me drink it. Nothing further. I just lie and cry until the night. From longing for one's own. From my betrayal. From injustice. I seemed to fall asleep at night. I woke up at 4 am, as I usually get up at home. Very nauseating. While I did all the procedures, I walked around. At 7 am they came and told me to go donate blood. I have good veins, I always tolerate it well. The first time I lost consciousness from donating blood. Maybe it’s because I’m hungry (I didn’t eat anything yesterday, I forgot), maybe it’s nerves, maybe it’s the effect of the medicine. They collected blood by laying him on the couch and piercing his other arm. Like this. The weakness was severe, they brought him to the ward. I passed out like dead and slept for 2 hours. At 9.00 they called us for breakfast. I got up. I really wanted to eat. But I couldn't. Be sick. I forced myself to eat 10 spoons of rolled oats, I needed strength. Sweet tea. Then I found 6000 steps in the ward. Then I was sent to the next building for fluorography. I took a walk, even went to the store to buy some water. I came and started writing this post. At 12.30 I was given another tablet. At 13.00 I had some lunch. Now I’m lying here with a really bad headache and that’s all for now...
Girls! I still want to support each of you!!! Do not give up!!! Appreciate what you have!!! And those who have yet to pass the test! Hang in there, girls!!!
I know that many more unpleasant and sad things will happen to me these days and I feel bad, very bad. But I know that they are waiting for me at home and love me!!! And how much I love and appreciate...
Happiness to you girls!!!