Neuroprotective therapy for chronic cerebral ischemia
New demographic trends today are of particular relevance in geriatric practice. In the second half of the 20th century, the age composition of the population changed significantly. Improvements in socio-economic conditions and the quality of medical care in industrialized countries have led to a significant increase in life expectancy. The result of this was a significant increase in the number of elderly and senile people. According to the 2010 All-Russian Population Census, about 47 million elderly people live in our country, 71.8% are people over working age [1]. The fastest growing age group in Europe is older people over 80 years of age.
The main group of patients visiting the clinic in our country are elderly people. Thus, for 1 patient aged 50 years and older there are from 1.7 to 3.6 diseases (while for people 70 years and older there are 5–7 diseases). The most common complaints with which elderly patients consult a doctor are associated with manifestations of cerebrovascular disease (insomnia, depression, cognitive disorders), exacerbation of chronic somatic diseases, and side effects from frequent and sometimes uncontrolled use of medications [1].
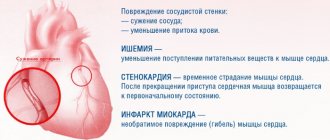
Cerebrovascular diseases (CVD) are one of the main causes of mortality and permanent disability in patients. According to the classification of cerebrovascular disorders adopted in our country in 1958, dyscirculatory encephalopathy (DEP) is the main clinical form of chronic cerebrovascular insufficiency [2–4]. Despite the fact that in the International Statistical Classification of Diseases and Related Health Problems, the IX and X revisions, DEP as a nosology is absent and it was replaced by the term “cerebral ischemia (chronic) (CHI)” - ICD-10, class IX “Diseases of the circulatory system” "(I00-I99), I67.8 - "Other specified cerebral vascular lesions: 1) acute cerebrovascular insufficiency of 5-bromo-2'-deoxyuridine (NOS) and 2) cerebral ischemia (chronic)", the term is still widely used "encephalopathy".
Definition. CCI is a special type of vascular cerebral pathology, caused by a slowly progressive diffuse disorder of the blood supply to the brain with gradually increasing various defects in its functioning [5]. DEP is a chronic progressive form of cerebrovascular pathology, characterized by the development of multifocal or diffuse ischemic brain damage [3, 4, 6, 7]. DEP, like stroke, is a cerebrovascular syndrome [2] - a syndrome of chronic progressive brain damage of vascular etiology, which develops as a result of repeated acute cerebrovascular accidents and/or chronic insufficiency of blood supply to the brain [8].
The clinical picture of DEP is characterized by [4]: 1) a progressive increase in cognitive impairment (decreased memory, attention, intelligence), reaching the level of dementia in the final stages, which is manifested by a combination of pronounced impairment of cognitive functions, personality changes with significant difficulty in normal social activity and the inability to continue work; 2) a gradual increase in emotional impoverishment, loss of interest in life; 3) gradual increase in coordination and walking disorders, destabilization of the tempo and rhythm of movements, tendency to falls; in severe cases, walking becomes impossible, despite the absence of paresis; 4) subcortical syndrome: oligobradykinesia, hypomimia, acheirokinesis, increased muscle tone of the extrapyramidal type (like parkinsonism syndrome); 5) pseudobulbar syndrome of varying severity: dysarthria, dysphagia, forced laughter and crying, symptoms of oral automatism; 6) decreased strength in the limbs, mild paresis with severe brain damage; 7) gradual appearance of disturbances in control of the function of the pelvic organs.
There are three stages of DEP: I - mild or moderate (compensation), II - severe (subcompensation), III - pronounced (decompensation) [9, 10]. For DEP Art. I typical [4]: complaints of increased fatigue, frequent headaches, irritability, moderate memory impairment (primarily operational), moderate decrease in performance, sleep disturbances, which are accompanied by fairly persistent objective disorders in the form of anisoreflexia, discoordination phenomena, mild oculomotor disorders, symptoms oral automatism. There are diffuse neurological symptoms, and the identified disorders are subsyndromal in nature [9]. The early stages of DEP are most characterized by vascular depression and emotional lability, and, as is known, emotional disorders can have an adverse effect on the cognitive sphere [8]. The gradual progression of the disease and worsening symptoms are a powerful factor in social maladjustment [1].
With inadequate treatment, DEP progresses and turns into stage II DEP, in which the noted diffuse neurological symptoms develop into a separate dominant syndrome, which most significantly reduces the patient’s professional and social adaptation [4, 9]. Anxious and depressive reactions appear, cognitive disorders worsen, mental production and volitional activity decrease, professional memory deteriorates, there is a viscosity of thinking, a narrowing of the range of interests, a decrease in criticism and a change in personality. Circadian rhythms are disrupted in the form of daytime sleepiness with poor night sleep. For DEP II Art. characteristic: deepening memory impairment and decreased attention function, increasing intellectual and emotional disorders, and a significant decrease in performance. Somewhat less frequently, there are complaints of chronic fatigue, headache and other manifestations of asthenic syndrome. In some patients, mild subcortical disturbances and changes in gait are detected (it becomes shuffling, mincing). Focal symptoms are more clearly manifested in the form of revitalization of reflexes of oral automatism, central insufficiency of the facial and hypoglossal nerves, coordination and oculomotor disorders, pyramidal insufficiency, and amyostatic syndrome [4].
With DEP stage III. the number of complaints decreases, which is due to a decrease in patients’ criticism of their condition, the severity of intellectual-mnestic and neurological disorders increases, unmotivated actions and inadequate reactions are observed, emotional disorders are characterized by a dysthymic-dysphoric mood background with irritability, dissatisfaction with others and weakness. This stage is characterized by: clearly defined discoordination, amyostatic, psychoorganic, pseudobulbar, pyramidal syndromes. Paroxysmal conditions are more often observed - falls, fainting, epileptic seizures. The main difference between DEP Art. III. from DEP II Art. is that with DEP stage III. In the clinic, several fairly pronounced syndromes are observed, while with DEP stage II. one of them clearly predominates [4, 9].
The relationship between the proportion of complaints and neurological symptoms at different stages of DEP is shown schematically in Fig.
Diagnosis of DEP is possible if the following criteria are met [4]: 1) objectively identified neuropsychological or neurological symptoms; 2) signs of CVD, including risk factors (arterial hypertension, hyperlipidemia, diabetes mellitus, heart rhythm disturbances, etc.), and/or anamnestic signs, and/or instrumentally confirmed signs of damage to cerebral vessels or brain matter; 3) evidence of a causal relationship between (1) and (2); 4) correspondence of the dynamics of neuropsychological and neurological deficits to the characteristics of the course of CVD (a tendency to progression with alternating periods of sharp deterioration, partial regression and relative stabilization); 5) correspondence of changes in the brain substance of vascular origin detected by computed tomography/magnetic resonance imaging with the leading clinical manifestations; 6) exclusion of other diseases that can explain the clinical picture.
Diagnostic criteria for DEP [11]: 1) presence of signs (clinical, anamnestic, instrumental) of brain damage; 2) the presence of signs of acute or chronic dyscirculation (clinical, anamnestic, instrumental); 3) the presence of a cause-and-effect relationship between disorders and the development of clinical, neuropsychological, and psychiatric symptoms; 4) clinical and paraclinical progression of cerebrovascular insufficiency.
Course of DEP and prognosis. There are stable, slowly progressive (with paroxysms and transient cerebrovascular accidents and without vascular episodes), paroxysmal, rapidly progressive course. Stable and slowly progressive are more typical for stage I DEP, which can last for 7–12 years. With a rapidly progressive variant of stage II or III DEP. develop in less than 5 years of illness. Clinical prognosis of stage III DEP. unfavorable, at this stage of the disease patients often need care, and sometimes are completely helpless in everyday life.
Cognitive impairment is a key manifestation of DEP, which largely determines the severity of the patient’s condition. They can serve as the most important diagnostic criterion for DEP and are perhaps the best guide for assessing the dynamics of the disease [2]. At the early stage of DEP, moderate neurodynamic disturbances predominate in the form of slowness, aspontaneity, decreased performance, exhaustion, and weakened concentration. However, such patients generally perform well on timed tests, consistent with mild cognitive impairment [2]. Further progression of the cognitive defect in DEP is associated with the development of dementia, in which cognitive deficit (regardless of motor and other symptoms!) leads to limitation of daily activity and at least partial loss of household independence [2].
Treatment of patients with DEP is a complex medical and social problem and, in fact, is limited to the therapeutic effect on the manifestations of DEP stages I and II. The main directions of management of these patients are reversing the developed decompensation of the pathological process, preventing the progression of the disease, including stroke, reducing the severity of cognitive disorders and neurological deficits [10, 11]. The most effective measure to prevent further progression of the disease is to influence vascular risk factors, primarily proper antihypertensive therapy, correction of carbohydrate and lipid metabolism, changes in vascular tone, increased cerebral perfusion, and improvement of brain tissue metabolism [2].
The basis of therapy aimed at improving the well-being of a patient with DEP and improving his quality of life are drugs that affect cerebral circulation at the microcirculatory level (vasoactive drugs) and drugs that improve metabolic processes in the brain (nootropic drugs). Vasoactive drugs improve blood supply to the brain by dilating the microvasculature. These include: phosphodiesterase blockers (such as pentoxfilline), including those of plant origin (Ginkgo biloba); calcium blockers, the effect of which is most pronounced if the blood flow is impaired in the vertebral arteries supplying blood to the brain stem; α-blockers acting on receptors of the vascular wall. Nootropic drugs can increase neuronal plasticity, thus increasing the adaptive capabilities of nerve cells and reducing their susceptibility to damaging factors by improving metabolic processes in neurons. Nootropic drugs have a positive effect on the higher integrative functions of the brain, facilitate learning processes and memory consolidation.
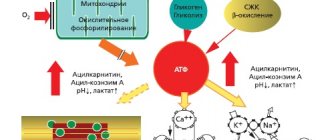
Today, neurotrophicity, neuroprotection, neuroplasticity and neurogenesis are considered fundamental neurobiological processes involved in the implementation of endogenous protective activity, as well as in attempts to counteract pathophysiological damaging mechanisms and stimulate endogenous repair. The classical concept of neuroprotection involves suppression of a specific pathophysiological mechanism using an appropriate drug [12]. The action of various drugs is aimed at enhancing the oxidation of glucose in mitochondria (Actovegin), inhibiting the oxidation of long-chain fatty acids, which increases the synthesis of adenosine triphosphoric acid (ATP) and neutralization of oxygen radicals, the production of which increases under ischemic conditions [13].
Today, there are several pharmacological groups of drugs with neurometabolic action. They can be roughly divided into classic ones, used for several decades to treat patients with cognitive impairment, and drugs that have relatively recently become widespread in the practice of rehabilitation of patients with vascular diseases of the brain, and were originally proposed for the treatment of Alzheimer's disease. The first group includes Actovegin, piracetam, pyriditol and Cerebrolysin [6]. One of the drugs that has a wide range of pharmacological effects (antihypoxic, antioxidant) is Actovegin, a drug that has a neurotrophic effect. Antioxidant and anti-apoptotic mechanisms of action underlie the neuroprotective properties of Actovegin. It not only improves glucose transport and oxygen absorption, but also stimulates their utilization, which improves oxygen metabolism even under hypoxic conditions.
Impaired cognitive functions (lack of attention, concentration, impaired ability to quickly orientate in a changing environment; decreased memory, especially for current events; slowness of thinking, rapid exhaustion during intense mental work, narrowing of the range of interests) is one of the most common manifestations of CVD. Treatment of cognitive impairment in DEP should, first of all, include measures to prevent further damage to cerebral vessels and brain matter, improve and long-term stabilization of cognitive functions, and correct other clinical manifestations of the disease [2]. On the 1st and 2nd stages. DEP cognitive impairment is present in 88% of cases. A wide range of nootropic and neuroprotective drugs are used to improve cognitive functions [8].
A double-blind, placebo-controlled study was conducted to evaluate the therapeutic effect of the drug Actovegin (tablet form containing 200 mg of the active substance) on mnestic-intellectual abilities in patients with CVD [14]. The study included 120 patients with CVD; the average age of the patients was about 67 years (60–72 years), and the average duration of the disease was 2.5 years. All patients were randomized into 3 groups: group 1 (n = 40), taking Actovegin 3 tablets 3 times a day; group 2 (n = 40) - placebo, of which: 20 patients took 2 tablets 3 times a day, 20 patients took 3 tablets 3 times a day; group 3 (n = 40) - Actovegin 2 tablets 3 times a day. The duration of therapy was 12 weeks, with assessment of the dynamics of the condition before the start of therapy, after 4, 8 and 12 weeks of treatment. To assess the clinical effectiveness of Actovegin, tests were used that assessed mnestic-intellectual functions (memory, synthetic and analytical abilities, concentration, comprehension). The study showed high effectiveness (92%) of 12-week therapy with Actovegin forte on mnestic-intellectual abilities in patients with CVD. Thus, the tablet form of Actovegin can be recommended for long-term outpatient treatment of elderly patients with DEP.
A multicenter study was conducted to evaluate the effectiveness of Actovegin in 1549 elderly patients with impaired cerebral functions [15]. The average age of the patients was 74.1 years. Patients included in the study received treatment according to a standard regimen: 2 weeks of intravenous injections of 10 ml of Actovegin solution, and then 4 weeks of oral administration of Actovegin film-coated tablets (2 tablets 3 times a day). Patients were examined before the start of therapy, two weeks after IV therapy and after 4 weeks of oral therapy. All baseline values were compared with results after 2 and 6 weeks of treatment, and results obtained after 2 weeks were also compared with results after 6 weeks of treatment. After 4 weeks from the start of Actovegin therapy, in 80% of cases, an improvement in the general condition was noted: a decrease in the severity (or cessation) of headaches, dizziness, anxiety and feelings of fear, improvement in memory and concentration (according to psychological testing). In 10.9%, undesirable effects of the drug were noted (feelings of heat and nausea), which were observed in the first, parenteral phase of therapy. The study shows the practical significance of combination therapy: start therapy with iv 10 ml of Actovegin to achieve a quick and good response, followed by continued oral administration of Actovegin forte tablets). The proposed treatment regimen with Actovegin can be recommended for long-term therapy of elderly patients with organic syndrome.
A number of clinical studies have shown that the use of the drug Actovegin has a positive effect on cognitive functions, improves psychological and behavioral reactions, and is also most effective for mild and moderate cognitive impairment. Currently, therapeutic regimens for the treatment of CVD have been developed and are widely used with the prescription of Actovegin: 10 ml (400 mg) per 200 ml of saline solution intravenously for 7–10 days, then 1–2 tablets (200–400 mg) 3 times /day orally for 1-2 months, in the presence of mnestic-intellectual disorders in the elderly - up to 12 weeks, 2-3 tablets 3 times a day. Repeated courses after 6–8 months [16].
In the prevention and treatment of moderate cognitive disorders of vascular origin, neurometabolic drugs have been shown to be highly effective, including the drug Ceraxon (citicoline) [17–19]. The drug has a targeted effect on the key links in the processes of death of nerve cells of vascular, traumatic, toxic and other etiologies. Since Ceraxon is a natural metabolite of biochemical processes in the body and combines neurotransmitter and neurometabolic effects in its spectrum of action. The most important of them is the activation of the biosynthesis of membrane phospholipids of brain neurons, primarily phosphatidylcholine. Citicoline can correct cognitive impairments already at the initial stages of their manifestations in patients with DEP [18]. Its use promotes regression of cognitive impairments and reduces concomitant emotional, affective and behavioral disorders. Cerakson is able to potentiate the effect of other drugs in the treatment of acute cerebrovascular pathology, including thrombolytics, antiplatelet agents and neurotrophics [17]. Prescription regimen for the drug Ceraxon: 1000 mg for 10 days IM or IV once a day, then Ceraxon solution 2 ml orally 3 times a day for 3 months [18] or according to the scheme [20]: daily at a dose of 3 ml 2 times a day for 6 months. The duration of neuroprotection can last up to 12 months.
Conclusion
Moderate and severe cognitive impairment of a cerebrovascular nature can serve as an equivalent to DEP and is detected in 3–16 people over 60 years of age [2]. The main thing in the treatment of a patient with stage I or II DEP. in an outpatient setting is to relieve the developed decompensation of the pathological process, prevent the progression of the disease, including stroke, reduce the severity of cognitive disorders and neurological deficits [21].
A number of studies have shown the high clinical effectiveness of administering the drugs Actovegin and Cerakson as long-term neuroprotection in patients with dyscirculatory encephalopathy.
Literature
- Shavlovskaya O. A. Medical and social aspects of the elderly / Articles of the All-Russian Scientific and Practical Conference with International Participation “Society and Health: Current State and Development Trends”. 2013, vol. 2, p. 365–371.
- Levin O. S. Use of Ginkgo biloba extract (EGb 761) for the treatment of cognitive impairment in dyscirculatory encephalopathy // Breast Cancer. 2009, vol. 17, no. 20, p. 1356–1361.
- Maksudov G. A. Dyscirculatory encephalopathy. In the book: Vascular diseases of the nervous system. Ed. E. V. Schmidt. M.: Medicine, 1975, pp. 501–512.
- Temnikova E. A. The use of the drug Omaron in the practice of a general practitioner when working with elderly patients // RMZh. 2009, vol. 17, no. 20, 1345–1356.
- Putilina M.V. Modern ideas about the treatment of anxiety and depressive disorders in chronic cerebral ischemia // RMJ. 2011, no. 9, p. 569–573.
- Polyakov I. A., Malozemov I. V., Stepanova N. S. Antioxidant therapy in the complex treatment of dyscirculatory encephalopathy // Terra Medica Nova. 2009, no. 4–5, p. 22–24.
- Shtulman D. R., Levin O. S. Neurology. Handbook of a practicing physician. M.: MEDpress-inform, 2008, 1025 p.
- Zakharov V.V., Lokshina A.B. Cognitive impairment in discirculatory encephalopathy // Breast Cancer. 2009, vol. 17, no. 20, p. 1325–1329.
- Dadasheva M.N., Podrezova L.A., Shuchalin O.G. et al. Algorithm for the treatment of dyscirculatory encephalopathy in patients with arterial hypertension in general medical practice // RMZh. 2009, vol. 17, no. 20, p. 1320–1324.
- Kadykov A. S., Shakhparonova N. V. Vascular diseases of the brain. Miklos, 2006, 192 p.
- Damulin I.V., Parfenov V.A., Skoromets A.A., Yakhno N.N. Circulatory disorders in the brain and spinal cord. In the book: Diseases of the nervous system. Guide for doctors. Ed. N. N. Yakhno, D. R. Shtulman. M., 2003. pp. 231–302.
- Shakhparonova N.V., Kadykov A.S. Antioxidant therapy for cerebrovascular diseases // Breast Cancer. 2010, vol. 18, no. 26, p. 1570–1572.
- Astashkin E.I. The influence of Actovegin on the energy metabolism of cells during ischemia. Proceedings of MMA im. I. M. Sechenov. M., 2009: 1–4.
- Jansen V., Bruckner G.V. Treatment of chronic cerebrovascular insufficiency using Actovegin forte tablets (double-blind, placebo-controlled study) // RMZh. 2002. T. 10. No. 12–13. pp. 543–546.
- Letzel H., Schicktiger U. Use of Actovegin in elderly patients with organic syndromes. Multicenter study of 1549 patients // Breast Cancer. 2003. T. 11. No. 25. P. 1428–1431.
- Shavlovskaya O. A. Use of Actovegin in neuroprotective therapy of patients with cerebrovascular diseases // Journal of Neurology and Psychiatry named after. S. S. Korsakova. 2013, v. 113, no. 6, p. 74–76.
- Boyko A. N., Kabanov A. A. Cytocoline: new possibilities of neuroprotection and pharmacotherapy for diseases of the nervous system // Farmateka. 2007, no. 15, pp. 42–48.
- Putilina M.V. Features of combined neuroprotective therapy for acute cerebrovascular accidents // Breast Cancer. 2009, vol. 17, no. 4, p. 261–267.
- Putilina M.V., Shabalina N.I. Possibilities of early correction of mild and moderate cognitive disorders in patients with dyscirculatory encephalopathy // Treating Doctor. 2010, no. 9, p. 100–103.
- Shavlovskaya O. A. Neuroprotective therapy of neurological deficit in cerebrovascular pathology // Practicing doctor today. 2012, no. 3, p. 39–44.
- Shavlovskaya O. A. Efficacy of antioxidant drugs in the treatment of mild and moderate cognitive disorders // Breast Cancer. 2013, vol. 21, no. 10, p. 476–480.
O. A. Shavlovskaya, Doctor of Medical Sciences
GBOU VPO First Moscow State Medical University named after. I. M. Sechenova Ministry of Health of the Russian Federation, Moscow
Contact Information
D. Molchanov*
Alzheimer's disease (AD) is a primary degenerative brain disease that usually occurs in people over 50 years of age and is characterized by a progressive decline in intelligence, memory impairment, and personality changes. The first description of the clinical picture of this pathology was made in 1907 by Alois Alzheimer, but deciphering the reasons for its development became possible only at the end of the twentieth century thanks to a series of complex immunopathochemical and genetic studies. Despite certain successes in the search for effective pharmacotherapy for AD, this disease still occupies one of the leading positions in the list of pathological conditions of the central nervous system, leading to severe disability and social maladaptation of patients. Given the trend towards increasing life expectancy in developed countries and the associated increase in the incidence of asthma, it should be expected that this problem will remain relevant in the near future. The program of the III National Congress of Neurologists, Psychiatrists and Narcologists of Ukraine has traditionally been rich in reports from leading scientists on the issues of providing assistance to patients with impaired cognitive functions, including those caused by processes of neurodegeneration. We bring to our readers an overview of presentations by authoritative European specialists recognized as experts in the field of studying the possibilities of preventing and treating Alzheimer's disease. Dr. Anton Alvarez (EuroEspes Biomedical Research Center, Spain) shared new experimental and clinical data regarding the pathogenesis of AD and the use of pharmacological neuroprotection in people with age-related cognitive impairment. To date, no possibility has been found to radically influence the process of progression of senile neurodegeneration, which manifests itself in the clinical picture of AD, despite significant advances in deciphering the mechanisms of formation of this pathology. Therefore, in modern pharmacotherapy of AD, the search is aimed at discovering new agents that can slow down the progression of this process. The potential for the prevention and treatment of AD today is associated with the use of therapeutic agents that can influence risk factors for the development of the disease, modify its course, and also reduce the degree of functional deficit. Knowledge of the main biomarkers of AD allows us to adequately assess the clinical potential of individual drugs in experiments. Thus, the main pathomorphological manifestation of neurodegeneration in AD is the deposition of β-amyloid (Ab) in senile plaques. β-Amyloid is formed from amyloid precursor protein, which is one of the proteins that make up cell membranes. Under normal conditions, the amyloid precursor protein is metabolized into several fragments, one of which is β-amyloid. The formation of β-amyloid is possible in the form of two forms - Aβ40 and Aβ42, which differ in the number of amino acid residues and weight. The heavier form of Aβ42 quickly forms an insoluble aggregate, and this form of amyloid is currently given special importance. The formation and deposition of β-amyloid, hyperphosphorylation of tau protein, accumulation of pathological proteins, and increased activity of oxidative enzymes in turn lead to hyperproduction of free radicals. The severity of cognitive impairment in AD corresponds to the severity of pathomorphological changes - the number of senile plaques, neurofibrillary deposits and lost synapses. Researchers judge the degree to which the process of Aβ deposition is slowed down under the influence of therapy by measuring the concentration of this substance in the cerebrospinal fluid, as well as using positron emission tomography. The death of neurons, accompanied by atrophy of certain areas of the brain, in AD can also be assessed by neuroimaging methods. The metabolic activity of the central nervous system structures is judged by studying the metabolism of glucose in the nervous tissue. The clinical effectiveness of pharmacotherapy for asthma is assessed using standardized scales that make it possible to determine the degree and rate of decline in cognitive functions, as well as the severity of non-cognitive symptoms during treatment. In this case, the duration of observation of patients for an objective assessment of the effect of the therapy should be at least 1-2 years. Separately, I would like to dwell on the immunotrophic mechanisms of asthma progression, one of which is associated with a decrease in the level of insulin-like growth factor (IGF-I) in the body and a simultaneous increase in the level of tumor necrosis factor (TNFα). Insulin-like growth factor influences the processes of neuroprotection, while tumor necrosis factor potentiates the processes of inflammation and neurotoxicity. The role of the imbalance of these endogenous substances in the pathogenesis of neurodegeneration has been confirmed in many experimental studies. Thus, in a study by Carro et al. (2002) showed that peripheral administration of insulin-like growth factor slows down the formation of β-amyloid in rodents, while tumor necrosis factor has an effect opposite to the effects of IGF-I. In 2006, the same team of researchers proved that blockade of IGF-I receptors in the choroid plexus causes AD-like neuropathy in mice. An increase in the level of TNFα against the background of a decrease in IGF-I in the blood plasma in mild cognitive impairment and AD was shown in our study (A. Alvarez et al., 2007). Thus, there are currently experimental prerequisites for the use of pharmacological agents that can eliminate immunotrophic imbalance in order to slow down the processes of neurodegeneration in AD. In this regard, the use of Cerebrolysin, a neuroprotector with proven clinical effectiveness, is of great interest. Cerebrolysin is a unique peptidergic drug with activity similar to that of natural neurotrophic factors. The neurotrophic properties of Cerebrolysin have been proven by the work of many researchers: S. Shimazu et al. (1992), T Satou. et al. (1993), E. Masliah et al. (1999), M. Hartbauer et al. (2001), Y. Tatebayashi et al. (2003). To date, an extensive evidence base has also been accumulated regarding the ability of Cerebrolysin to slow down neurodegeneration in AD. In the works of Akay et al. (1992) demonstrated its neurotrophic effect on cholinergic neurons, which are especially susceptible to degeneration in this pathology. Albretch et al. in 1993, they showed the ability of the drug to increase the survival of neurons in isolated culture, as well as to activate dendritic branching. The ability of Cerebrolysin to slow down neurodegeneration and apoptosis induced by β-amyloid and lipopolysaccharides (LPS) was proven in our experimental work by A. Alvarez et al. (1999) and the following year, Cerebrolysin was shown to suppress Aβ- and LPS-induced microglial activation. In 1999, Lombardi et al. demonstrated a decrease in the production of pro-inflammatory interleukin 1a caused by lipopolysaccharide activation of microglia during Cerebrolysin administration. In a study by Rockenstein et al. (2002), conducted on rodents, showed that the administration of Cerebrolysin significantly reduces the deposition of pathological amyloid in the tissue of the frontal cortex of the brain and preserves the integrity of synapses compared to placebo. In a later work (2006), the same authors were able to explain the mechanism by which this effect of the drug is realized. Long-term use of Cerebrolysin slows down the phosphorylation of amyloid precursor protein (APP) in the β-amyloid maturation chain, thereby interrupting the pathological process, the logical conclusion of which is the loss of synaptic connections between gray matter neurons and the accumulation of pathological proteins. In addition, we have shown that Cerebrolysin has a dose-dependent ability to restore the immunotrophic balance of nervous tissue by normalizing the levels of IGF-I and TNFβ, the role of which in the pathogenesis of AD was discussed above, with the most pronounced effect observed when using doses of 30 and 60 ml . This property of the drug can also change the course of asthma, leading to a slowdown in its progression. Thus, preclinical evidence of Cerebrolysin’s ability to modify the course of AD includes the results of experimental studies demonstrating its ability to reduce amyloid deposition in nervous tissue, slow down neuronal degeneration and synapse loss, and reduce microglial activation. Today there is also convincing clinical evidence of the effectiveness of this neurotrophic drug in AD. In the works of Bae et al. (2000), Moessler et al. (2000), Ruether et al. (2001), Rainer et al. (2002), Panisset et al. (2002), as well as our team of authors, showed the ability of Cerebrolysin to improve cognitive functions, reduce the severity of non-cognitive symptoms, increase the activity of daily life and global functioning of patients with age-related dementia. This year, a study we conducted together with Professor Muresanu (Romania) demonstrated the phenomenon of increasing the index of bioelectrical activity of the brain due to increased power of the a and b rhythm during Cerebrolysin therapy, and also showed a direct correlation between this indicator and improvement in cognitive functions in patients with vascular dementia when assessing mental impairment using the ADAScog scale. A double-blind, placebo-controlled study of three dosage regimens of Cerebrolysin in 279 patients with mild to moderate asthma (A. Alvarez et al., 2006) demonstrated that the severity of cognitive impairment, assessed by the ADAScog scale, was most reduced with the 10 ml dose. /day for 12 weeks of therapy, while dosages of 30 and 60 ml/day with the same duration of therapy did not significantly affect the cognitive sphere of patients. Similar therapeutic differences in Cerebrolysin dosages in terms of the degree of influence on cognitive functions in favor of a dose of 10 ml/day are provided by a systematic review of the largest library of evidence-based databases, the Cochrane Database (table). At the same time, the ability of Cerebrolysin to improve cognitive function is comparable, and in some studies exceeds that of cholinesterase inhibitors. When analyzing the ability of Cerebrolysin to increase the daily activity of patients, it was noted that doses of 10 and 30 ml/day had a comparable positive effect. At the same time, the maximum and significant decrease in the severity of neuropsychiatric symptoms was observed in the group of patients receiving a dose of 60 ml/day. Summarizing the data obtained in our study, it should be noted that the positive clinical effect in asthma is inherent in the entire spectrum of therapeutic doses of Cerebrolysin, however, the severity of this effect varies in relation to individual indicators of the neurofunctional status of patients. When analyzing the safety of Cerebrolysin, there were no significant differences between the three therapeutic groups (10, 30 and 60 ml/day) and the placebo group in the frequency of side effects, the use of auxiliary drugs and changes in laboratory parameters. It should also be noted that the therapeutic benefits of Cerebrolysin were maintained for 3 months. after completion of the course of treatment, thereby confirming the modifying and stabilizing effect of Cerebrolysin on the course of asthma. The results of the study allow us to draw a conclusion about the feasibility and safety of using this neuroprotector in the treatment of mild to moderate AD. The analysis of the possibilities of pathogenetic pharmacotherapy for asthma was continued by Professor Dafin Muresanu (Department of Neurology University of Medicine and Pharmacy, Cluj Napoca, Romania). The pathological process in AD is a special type of neurodegeneration, characterized by the progressive loss of presynaptic terminals of neurons, which begins in the hippocampus and gradually spreads to most areas of the cerebral cortex. It is the loss of synaptic connections that is the morphological substrate of the entire continuum of cognitive (from mild mnestic deficit to severe dementia) and motor disorders. The neuromorphological pattern of AD corresponds to a clinical pattern called retrogenesis, that is, the reverse development of the functions of a person’s higher nervous activity from a state of maturity to a status characteristic of early childhood. The concept of retrogenesis has received much confirmation. Thus, the picture of metabolic activity of the brain in a patient in the late stage of asthma when performing positron emission tomography is not much different from that in a normally developing infant. The fundamental causes of Alzheimer's-type neurodegeneration lie in the molecular chemistry of nervous tissue. The key point in the pathogenesis of AD is a genetically determined defect in the processing of intracellular proteins, leading to the formation and accumulation of their neurotoxic forms (b-amyloid, namely Ab42) in neurons. Numerous plaques consisting of deposits of pathological amyloid cause degeneration of neurons and their processes. Additional factors contributing to neuronal death include free radical oxidation, excitotoxicity, chronic inflammation, and pathological apoptosis. The progressive decrease in neuron density and atrophy of brain structures up to a certain point in time is compensated by the continuous process of regeneration of nervous tissue, the components of which are neurotrophism, neuroplasticity and neuroprotection. Neurotrophism determines the processes of proliferation, migration, differentiation and survival. Neuroplasticity is characterized by constantly occurring regeneration processes in cases of natural or pathological damage, while neuroprotection includes a variety of mechanisms directed against aggressive factors. The goal of modern pharmacotherapy for AD is to potentiate protective and neuroregenerative mechanisms while simultaneously inhibiting factors that contribute to the loss of neuronal connections. Current treatment options for patients with asthma include three strategies: symptomatic, disease-modifying and preventive. The first includes the use of anticholinesterase drugs that facilitate the transmission of nerve impulses at cholinergic synapses, as well as modulators of the glutamatergic system, without this group of drugs influencing the course of the disease. The second is to prescribe natural antioxidants (vitamin E, Ginkgo biloba extract), activators of endogenous neurotrophic factors, inhibitors of β-amyloid precursor protein synthesis and other agents that affect the pathogenesis and progression of dementia, most of which are under experimental study. Prevention of dementia is an even more complex area associated with the correction of risk factors and including the treatment of arterial hypertension, antihyperlipidemic therapy (effects on the vascular causes of progressive cognitive dysfunction), the use of anti-inflammatory drugs, hormone replacement therapy in women, etc. Promising developments are being carried out in the direction of the synthesis of universal molecules that combine all three treatment strategies. It is possible that in the near future, drugs will be created that have the properties of several classes of drugs, for example, cholinesterase inhibitors, selective monoamine oxidase inhibitors, M2 receptor agonists, antioxidants and neuroprotectors. But until this potential is revealed, the most promising direction in AD therapy remains the use of drugs with neurotrophic and neuroprotective properties. However, the results of scientific research in this direction also cannot be considered unambiguous. To date, more than 160 neurotrophic factors and growth factors with neurotrophic properties have been described, and profiles of their deficiency in various neurodegenerative diseases have been experimentally compiled. Dozens of cytoprotectors are also known, which, unfortunately, have not confirmed their clinical effectiveness in AD (A. Lise Labiche, C. James Grotta, 2004). It is quite obvious that by interrupting only one reaction of the most complex pathochemical cascade of this disease, it is impossible to significantly slow down the process of neurodegeneration and, accordingly, influence the clinical course of dementia. Therefore, the solution should again be sought in the use of targeted drugs, a prominent representative of which is Cerebrolysin - a combination of a number of neurotrophic and neuroprotective factors of natural origin (low molecular weight neuropeptides, phospholipids, neurotropic vitamins, essential microelements). The prerequisites for the use of Cerebrolysin in dementia are associated with the presence of specific mechanisms in this complex drug to slow down the process of neurodegeneration. The key point is the ability of cerebrolysine to inhibit the phosphorylation of the protein of the precursor of the pathological β-amyloid, confirmed in experimental studies. To date, the evidence base for the clinical use of cerebrolysin as a disease-modifying agent for BA includes research results conducted independently by various groups in many countries of the world (E. Ruether, 1994, 2001; M. Rainer, 1997; Sy. BAE, 2000; S. Xiao, 2000; M. Panisset, 2000; A. Alvarez, 2003, 2006). The encouraging data of six completed clinical studies confirming the ability of Cerebrolysin to modify the course of BA, combined in a special review of the US Department of Health (US Department of Health and Human Services), dedicated to the pharmacological treatment of dementia. It should be noted that this official document objectively reflects the point of view of the authoritative organization experts for the prospects for the use of modern neuroprotectors for the prevention and therapy of dementia, and the data related to the use of cerebrolysin demonstrate the increasing interest of researchers and practitioners in this well -known drug.
* Health of Ukraine 2007.
Modern concepts of neurocytoprotective therapy
Currently, both pharmacologists and doctors consider neurocytoprotectors as drugs that have the properties of increasing the survival of the neuronal cell cluster (a set of neurons, glial cells and their receptor-synaptic apparatus) under conditions of acute and chronic ischemia/hypoxia and other pathological influences. This group of drugs is not united by a single mechanism of action, but only by a name that translates as “protection of nerve cells,” and since we are talking specifically about nerve cells, today both this name and the drugs themselves have firmly entered into the clinical practice of only neurologists [ 1-3]. This significantly lengthens the path to complete and effective neuroprotection for patients who turn to internists, cardiologists, vascular surgeons and general practitioners with vascular problems [3]. Meanwhile, the mechanisms of not just neuro-, but, above all, cytoprotective action of many of these drugs make it possible to successfully use them in patients with vascular comorbidity leading to progressive cerebral insufficiency, as is the case with pathology of the heart, peripheral vessels, arterial hypertension, systemic diseases accompanied by endothelial dysfunction. All these pathological conditions lead to acute or chronic cerebral hypoperfusion (ischemia) and, consequently, to disorders of tissue metabolism [3].
The effectiveness of cytoprotectors acting on the basis of different mechanisms can be explained by a large number of ischemic imbalances (or autocoidoses), so named because their cascade reactions involve a large number of autocoids - disregulators of intracellular and interstitial homeostasis, which are produced by the body itself in response to primary hypoxia/ischemia. Metabolic, oxidative, mediator, cytokine, necrobiotic and apoptotic imbalances during acute or chronic ischemia occur at different rates, but ultimately lead to the gradual death of areas of brain tissue of different sizes - large foci in strokes, lacunar foci in chronic cerebral ischemia (CCI) and “small” strokes [1, 3]. It is known that individual parts of the ischemic cascade can be slowed down or blocked by neurocytoprotection—timely interventions aimed at cerebral damage caused by ischemia to prevent and/or correct functional and morphological changes in the cerebral and somatic systems of the body [3].
The relevance of the widespread use of neurocytoprotective therapy is associated with the observed increase in the number of patients with acute and chronic cerebral ischemia, atrophic and neurodegenerative processes caused by stroke, stenotic processes in the brachiocephalic arteries (BCA), diabetes mellitus, arterial hypertension, and Alzheimer's disease in recent years. And if acute vascular cerebral ischemia causes high mortality and rapid disability, then chronic cerebral ischemia leads to the steady development of dementia and a decrease in the quality of life of both elderly and middle-aged patients, including those of working age, and their loved ones [4], which in turn results in colossal socio-economic losses and requires the development of more effective means and methods of treatment and prevention [4].
Post-ischemic cerebral destruction as a means of treatment and prevention can be counteracted by a real “barrier”, which can be neuroplasticity and neuroregeneration. It was the creation of drugs with neurotrophic action several decades ago that laid the foundations for neuroprotective therapy. In those years, only one neuroprotector was widely known on the global and domestic pharmacological markets - Cerebrolysin. For both doctors and a huge number of patients, Cerebrolysin has been and remains a proven drug with high clinical effectiveness. To date, the drug has successfully passed the time evaluation. Due to its high efficiency, low number of side effects, successful use in neurological diseases of various origins (CCI, stroke, traumatic brain injury, moderate cognitive impairment, vascular dementia, Alzheimer's disease), Cerebrolysin occupies an important place in the list of modern neuroprotectors. The long-term “good clinical history” of the drug is today confirmed not only by numerous clinical studies, but also by experimental data, as well as multicenter double-blind placebo-controlled studies in recent years, conducted in compliance with all standards of good clinical practice (GCP) [5-12].
The development of Cerebrolysin as a drug intended for peptidergic neuroprotection was based on the pharmacologically correct way of using a protected complex of peptides and amino acids that have a neurotrophic effect. The neuroprotective effect of the peptide-amino acid complex created by Austrian pharmacologists is realized by its positive effect on several stages of post-ischemic neuronal damage [8]. After all, neuropeptides, like mediators, cytokines, hormones and other waste products of the body itself, are involved in the transmission of biologically important information to cells, facilitating extra-, intracellular and interstitial interactions, and also contribute to the activation of repair and regenerative cellular mechanisms. Most neuropeptides are capable of activating the formation of various growth factors (such as NGF) and their receptors (BDNF, Trk, etc.). Moreover, peptidergic agents are able to influence “deep” processes, not only plasmon, but also the cell nucleus, regulating the dynamic balance of the “scales” of intracellular homeostasis, on one side of which there is cell proliferation, and on the other - apoptosis. As a typical representative of the class of neuropeptides, Cerebrolysin has an inhibitory effect on the formation of FAS ligands - signaling systems that activate apoptosis, which provides protection and compensation for the functions of neurons and glial cells, stimulates the formation of new neuronal connections and provides both the cells themselves and their receptor activity trophic support [1-3, 8, 9]. Modern ideas associate the main mechanism of the molecular action of Cerebrolysin with the prevention of apoptosis - “programmed” cell death [3, 8].
Cerebrolysin is a naturally balanced mixture of 15% low molecular weight peptides (up to 10,000 D) and a set of essential amino acids and oligopeptides, obtained from porcine brain through specialized enzymatic processing. The neurotrophins contained in the drug exhibit an effect similar to the effects of neurotrophic factors, and amino acids, which make up up to 85% of Cerebrolysin, become part of the nutrient medium necessary for the survival of neurons. Most of the amino acids contained in the drug act on NMDA receptors, which mediate the transmission of nervous excitation through synapses, or are neurotransmitters (glutamate, aspartate) or their precursors. They can also modulate receptor functions - glycine, serine, arginine and lysine. Studies of the chemical composition have confirmed that Cerebrolysin contains more than 100 short peptides and proteins with a molecular weight of up to 5800 D. Among them, the presence of physiologically active peptides was revealed: thyroliberin, glutathione, enkephalin and collagen [3,6-11]. The drug also contains individual amino acids or small oligopeptides, which are individual blocks and amino acid “motifs” of neurotrophins, which determine its therapeutic properties and the variety of neurotrophin-like effects [3,11].
The discovery of nerve growth factor in the 50s of the last century and interest in the study of neurotrophins gave rise to the idea of neurotrophic therapy and partially explained the mechanisms of action of Cerebrolysin, which consist in the activation and proliferation of glial cells and early differentiation of cortical structures of the brain [5-8, 11 ]. The importance of these observations became apparent following classic studies demonstrating in vivo that administration of Cerebrolysin to neonatal rats stimulates the growth of hippocampal neurons, promoting dendritic branching and increasing the number of synaptic contacts [8, 9, 11, 12]. Moreover, the modern concept of preventing aging using “small peptides” suggests the possibility of correcting age-related changes with substances that activate tyrosine kinase receptors in brain structures, which specifically include Cerebrolysin [9].
The issue of effective dosages of Cerebrolysin has been debated for a long time, until numerous clinical studies have established that the effects of Cerebrolysin are dose-dependent. Evidence of this dependence was found during experimental work, during which it was shown that when Cerebrolysin was incubated with antibodies to growth factors, a specific dose-dependent increase in binding to neurotrophic factors was revealed - CNTF, GDNF, IGF-1(2) and ciliary neurotrophic factor (CNTF ). Recalculation of doses in clinical settings showed that to achieve optimal effect, the dose of Cerebrolysin must be large. And when studying the “ladder” of doses (0.5, 2.5 and 5 mg/kg) in experiment and clinic, it turned out that the effective dose of the drug is 2.5 mg/kg per day [3, 7-14].
More than 3,000 patients took part in clinical studies of Cerebrolysin conducted over the years. In a large number of studies, significant positive clinical results were obtained with the use of Cerebrolysin, expressed as an improvement in the general condition of patients, expansion of social contacts, increased independence from outside help and an increase in the level of intellectual capabilities. According to multicenter trials of Cerebrolysin, in patients in the acute period of carotid ischemic stroke, when prescribed Cerebrolysin in doses of 30 ml per day, a more rapid regression of neurological symptoms, a significant improvement in functional recovery and self-care skills in the long-term post-stroke period were recorded. The latest randomized, multicenter, double-blind, placebo-controlled study of the effectiveness of Cerebrolysin in ischemic stroke, CASTA, revealed a significant reduction in mortality and a more rapid regression of focal symptoms in patients with severe disease at a daily dosage of 20 ml. Similar studies have shown that a dose of 50 ml per day is even more effective and well tolerated [8, 9, 11]. Systemic effects of Cerebrolysin were also revealed: it was noted that the drug potentiates the effect of hypothermia and reduces the incidence of purulent-septic complications in ischemic stroke.
A number of studies have shown the effectiveness of Cerebrolysin treatment for mild cognitive impairment syndrome in patients aged 40-50 years based on the dynamics of changes in the Tinetti, Mattis, ADAS-Cog and other scales. A course of treatment for 20 days at a dose of 10 ml of the drug intravenously resulted in the same as immediately after completion of the course, or delayed—after 3 months—to a significant improvement in overall cognitive status, motor activity, and a decrease in functions, memory disorders and attention [15]. The positive effect of therapy was also established in patients with vascular dementia [16-21]. Treatment with Cerebrolysin contributed to the improvement of cognitive manifestations and normalized EEG parameters associated with cognitive activity of the brain, including a decrease in θ- and δ-waves and an increase in α-waves. After 3 and 6 months, scores on the MMSE were significantly higher in patients receiving Cerebrolysin compared to controls.
Fundamental research in recent years has made it possible to clarify the mechanisms of the multicomponent action of Cerebrolysin. Among these mechanisms, a major role is played by protection from oxidative stress and apoptosis. Thus, a comparative study of the effect of Cerebrolysin on microglia [22, 23] showed that the administration of the drug makes it possible to block inflammatory changes in glial cells that stimulate apoptosis. Direct evidence of the antiapoptotic effect of Cerebrolysin was obtained by studying cultured cortical neurons. Using a model of cytotoxic stress caused by a low serum content in the medium, a stimulating effect of Cerebrolysin on the axonal growth of telencephalon cells was established, which was most pronounced on days 4-8 of embryo development. The drug protected neurons from degeneration simulated in an artificial environment with a limited amino acid content. In these experiments, under the influence of Cerebrolysin, the number of cells with chromatin destroyed in the nucleus decreased [24].
Cerebrolysin, by stabilizing the level of calcium ions in cells, helps maintain protein synthesis and prevents neuroapoptosis caused by glutamate. The duration of the protective effect of the drug was monitored from 48 hours to 2 weeks, illustrating the wide possibilities of its use both within the “therapeutic window” and beyond. Control of DNA destruction showed a higher anti-apoptotic activity of Cerebrolysin in comparison with the neurotrophic factors BDNF, FGF-2 or the amino acid fraction. Cerebrolysin tripled the number of apoptotic cells of the P12 pheochromocytoma culture after stress exposure, and its addition to the tissue culture both before and after glutamate poisoning sharply weakened the cytomorphological picture of DNA destruction of neuronal nuclei, providing their protection from apoptosis [25,26].
Recent experimental studies [12, 26] have shown that cerebrolysin potentiates neurogenesis during neuronal ischemia through several mechanisms. The specificity of the effect on stem cells from non-ischemic and ischemic areas of the brain was demonstrated by drug dose-dependent proliferation effects. Under the influence of Cerebrolysin, the number of TUNNEL-positive cells at the border of the ischemic zone decreased. It is important in the light of consideration of the neurotrophic mechanisms of action of Cerebrolysin that under its influence the system of signaling proteins PI3K/Akt, coupled with tyrosine kinase receptor signaling, typical of neurotrophins, was activated.
A large number of clinical and experimental studies have demonstrated the effectiveness of Cerebrolysin in the treatment of Alzheimer's disease. Thus, the combination of Cerebrolysin + donepezil was used in a randomized multicenter study in patients with mild and moderate forms of Alzheimer's disease who received cyclic courses of therapy with the addition of aspirin for 24 weeks [27]. The results showed the effectiveness of this combination, although the number of side effects was slightly higher than with monotherapy. A series of clinical studies among patients with dementia of various etiologies, conducted since the mid-80s, showed that intravenous infusions of Cerebrolysin at a dose of 30 ml per day (a course of 28 days) led to a noticeable improvement in cognitive indicators [28].
Research by the group of E. Ruether et al. [29–31] were performed in patients with Alzheimer's disease. According to the general clinical impression and comparative assessment of the effectiveness of therapy on the ADAS-cog scale (Alzheimer's Disease Scale-cognitive subpart), significant improvements in performance were noted in the group receiving Cerebrolysin compared to placebo. It should be noted that the clinical effectiveness of therapy increased as the clinical picture of the disease became more severe, i.e. the result was similar to data obtained in a study of the effectiveness of Cerebrolysin in acute stroke. Thus, in patients with severe disease, according to Clinical Global Impression (CGI) and ADAS-cog indicators, by the end of the course of treatment, an increase in CGI indicators by 65% (versus 24.5% for placebo) and 4.1 times on the ADAS-cog scale was detected. cog. A repeated course of Cerebrolysin therapy, carried out 8 weeks after the end of the first course, showed high maintenance efficacy of Cerebrolysin, which differed significantly from the results in the placebo group.
Another study [32] confirmed significant improvements in CGI and ADAS-cog scores, as well as the Disability Assessment for Dementia. The effect of Cerebrolysin was maintained after discontinuation of therapy for at least an additional 12 weeks. Subsequent studies conducted in randomized, double-blind, placebo-controlled trials confirmed the effectiveness of long-term (24 weeks) Cerebrolysin therapy in mild to moderate forms of Alzheimer's disease [33]. A meta-analysis of 6 randomized trials in the treatment of mild and moderate stages of Alzheimer's disease [34] reliably confirmed the positive effect of Cerebrolysin (intravenous infusion of 30 ml of the drug for 4 weeks), assessed according to the main clinical tests.
The modern concept of “mild cognitive impairment” (MCD) provides a preventive approach to the treatment of dementia. And since 15-20% of the population aged 65 years show signs of MCS, which gradually transforms into dementia, the problem seems especially urgent. The use of Cerebrolysin in the treatment of MCS has brought quite significant results. During 2 years of therapy - repeated courses every six months, during which patients received 20 infusions of 30 ml of Cerebrolysin - a significant improvement in cognitive indicators was revealed [35].
Clinical and laboratory evidence for the high effectiveness of Cerebrolysin was obtained in recent years by the group of A. Alvarez et al. [36]. A study of patients with Alzheimer's disease and ISS syndrome revealed increased levels of TNF-a and decreased IGF-1 in the blood serum of these patients. A double-blind, randomized study (Cerebrolysin infusions of 10, 30 and 60 ml for 12 weeks) found a decrease in TNF-ct levels and a significant increase in IGF-1 in the blood serum of patients with Alzheimer's disease. The decrease in TNF-a went in parallel with the normalization of the cognitive status of the patients. The increase in IGF-I content was significantly significant at a dose of Cerebrolysin of 50 ml and correlated with a significant improvement in the neurological status of patients. These results are the first to link the therapeutic effect of Cerebrolysin to changes in the levels of neurotrophins in the blood of patients with cognitive disorders.
As is known, Alzheimer's disease is characterized by changes in the substructures of the glutamate receptor (GluRl) in the hippocampus, which lead to disruption of synaptic interaction. Cerebrolysin administered to “aged” rats increased the density of GluRl in the studied regions of the hippocampus, which caused positive changes in the results of tests of behavior, learning and memory in experimental animals. In a series of experimental studies performed on 5-month-old mice with a transgenic model of Alzheimer's disease [37], it was found that Cerebrolysin inhibited the formation of amyloid deposits in the frontal cortex, reduced the level of the A-B1-42 peptide, stimulated the regeneration of synapses and limited DNA fragmentation in the nuclei neurons. These effects were accompanied by an improvement in the synaptic consolidation of neurons in the frontal cortex and the elimination of deficits in learning and memory indicators. The protective effect of Cerebrolysin also related to the attenuation of perivascular microgliosis and astrogliosis and was illustrated by reduced expression of markers characteristic of cerebrovascular amyloidosis. Using a new genetic model of neurofibrillary and neurodegenerative pathology, disorders were induced in APP transgenic mice by introducing a tau-containing construct into the mutants. Against this background, treatment with Cerebrolysin led to a significant decrease in the level of tau phosphorylation, protecting hippocampal cells from destructive changes. In a culture of progenitor cells of the rat dentate nucleus, fibroblast growth factor (FGF-2) imitated elements of neurodegenerative phenomenology: it dose-dependently reduced the level of the cytoskeletal protein MAP2 and increased the content of the tau protein. Cerebrolysin administered intraperitoneally to animals, on the contrary, stimulated the differentiation of activated hippocampal progenitor cells and reduced signs of apoptosis. Studies on nerve cell cultures have led to the general conclusion that Cerebrolysin promotes: 1) modulation of the microenvironment of developing hippocampal cells; 2) increased neurogenesis; 3) functional maturation of the neuronal network due to the inhibitory effect on apoptotic processes. In the work of E. Rockenstein et al. [37], cerebrolysin administered to transgenic mice for 1 and 3 months significantly increased the number of neuroblasts in the subgranular zone of the hippocampus, as well as nuclear antigens of neuronal proliferation, which indicated stimulated neurogenesis. Thus, Cerebrolysin therapy promotes the activation of neurogenesis in mice with model transgenic APP pathology due to participation in plastic compensation.
One of the significant mechanisms of cerebrolysin's pleiotropic activity is associated with stimulation of neurogenesis. The first publications on Cerebrolysin demonstrated the effect of brain hydrolysates on the growth of nerve fibers in culture [5-8]. The results were obtained, the value of which is becoming increasingly obvious from the standpoint of modern neurobiology, based on ideas about the role of neurogenesis in the plastic support of brain functions. Cerebrolysin has been found to accelerate brain development in young rats; this phenomenon is accompanied by activation and proliferation of glial elements and early differentiation of cortical brain structures. Under physiological conditions, as found in these studies, stimulated neuroblasts from the subventricular region migrated to the olfactory region, where they differentiated into neurons [38]. Under conditions of focal ischemia, neuroblasts migrated to the border regions of ischemic damage, replacing damaged cells [39, 40].
Based on the above review of clinical and experimental data, it can be stated that the therapeutic effect of Cerebrolysin is determined by its ability to protect cerebral structures in ischemic and neurodegenerative pathologies by: 1) counteracting oxidative and apoptotic processes; 2) organization of new synaptic connections and activation of neuroplasticity; 3) correction of pathochemical reactions in cells and parts of the brain affected by the pathological process; 4) blockade of microglial activation and weakening of pro-inflammatory reactions in the vascular endothelium and nervous tissue; 5) stimulation of the activity of neuronal stem cells, their transformation, differentiation and migration to damaged areas of the brain. In addition, modern fundamental research makes it possible to consider Cerebrolysin as a means for maintaining and improving mental functions in conditions of “normal” aging and, most importantly, for preventive therapy of aging in general [9].
Thus, Cerebrolysin is a multifunctional drug that uses evolutionarily developed general biological repair mechanisms, which ensure the success of its use in various pathological conditions.
Literature
- Afanasyev V.V., Rumyantseva S.A., Lukyanova I.Yu. and others. Neuroprotection in ischemic stroke. Mat. conference "Emergency Medical Care, 2009". St. Petersburg2009.
- Rumyantseva S.A., Afanasyev V.V., Silina E.V. Pathophysiological basis of complex neuroprotection. Journal of neurol and psychiat 2009; 109:3:64-68.
- Rumyantseva S.A., Stupin V.V., Afanasyev V.V. and others. Critical conditions in clinical practice. M: MIG. Medical book 2011; 752.
- Skvortsova V.I., Stakhovskaya L.B. Epidemiology of stroke in the Russian Federation. Proceedings of the scientific and practical conference “Acute cerebrovascular accidents”. Irkutsk 2011; 7-14.
- Sommer N., Quandt J. Zur Wirkung cincs Hirnhydrolysatcs auf zcntral-ncrvocsc Strukturcn untcr Bcrucksichtigung clcktronoptischcr Ergcbnissc. Schwciz Arch Ncurol Ncurochir Psychiat 1973; 112: 373-386.
- Lindner G., Grosse G., Mutinies H., Kirsche W. Effects of brain extract and hydrolysate on nerve tissue in vitro. Z Mikrosk Anat Forsch 1975; 89:5:815-823.
- Ukraintseva SV, Arbeev KG, Michalsky AI, Yashin AI Antiaging treatments have been legally prescribed for approximately thirty years. Ann NY Acad Sci 2004; 1019:64-69.
- Gromova O A., Tretyakov V.E., Moshkovsky S.A. and others. Oligopeggid membrane fraction of cerebrolysin. J Neurol Psychiat 2006; 106:7:68-70.
- Gomazkov O.A. Brain aging and neurotrophic therapy. M: Ikar Publishing House 2011; 180.
- Crook T.N., Ferris SH, Alvarez XA. et al. Effects of N-PEP-12 on memory among older adults. Int Clin Psych op harmacol 2005; 20:2:97-100.
- Chen H., Tung YC, Li B. et al. Trophic factors counteract elevated FGF-2-induccd inhibition of adult neurogencsis. Ncurobiol Aging 2007; 28: 1148-1162.
- Zhang Ch., Chopp M., Cu Y. et ah Ccrcbrolysin enhances neurogencsis in the ischemic brain and improves functional outcome after stroke. J Ncuro-scicncc Research 2010; 88: 15: 3275-3328.
- Tatebayashi Y., Lee Mh., Li L. et al. The dentate gyrus neurogencsis: a thcr-aupcutic target for Alzheimer disease. Acta Ncuropathol 2003; 105: 225-232.
- Satou T, Itoh T, Ohde H. et al. Ncurotrophic effects of FPF-1070 (Ccrcbrolysin) on cultured neurons from chicken embryo dorsal root ganglia, ciliary ganglia, and sympathetic trunks. J Neural Transm 2000; 107: 11: 1253-1262.
- Damulin I.V., Koberskaya K.N., Mkhitaryan E.A. The effect of Cerebrolysin on moderately severe cognitive impairment in discirculatory encephalopathy (clinical and electrophysiological study). Journal of neurol and psychiat 2007; 107:5: 32-38.
- Muresanu DF, Alvarez XA, Moessler N. et al. A pilot study to evaluate the effects of Ccrcbrolysin on cognition and qEEG in vascular dementia: cognitive improvement correlates with qEEG acceleration. J Ncurol Sci 2008; 267: 1-2: 112-119.
- Vereshchagin N.V., Suslina Z., Timerbaeva S.JI. et al. Treatment and prevention of cognitive dysfunction in patients with arterial hypertension and atherosclerosis: results of a randomized, double-blind, placebo-controlled trial of Cerebrolysin. Ter Arch 2001; 73:4:22-27.
- Xiao S., Yan N., Yao P. and the CCRCbrolysin Study Group. Efficacy of FPF 1070 (Ccrcbrolysin) in Patients with Alzheimer's Disease. Clin Drug Invest 2000; 19:43-53.
- Tapu M., Bicu D., Tapu F., Stovicek O. The efficacy of ccrcbrolysin in vascular dementia. J Neurological Sciences 2009; 283:1-2:286.
- GuekhtA.B., Moessler H., Novak PH., Gusev EI On behalf of the CCRCbrolysin Investigators. Ccrcbrolysin in Vascular Dementia: Improvement of Clinical Outcome in a Randomized, Double-Blind, Placebo-Controlled Multiccntcr Trial. J Stroke Ccrcbrovasc Dis 2010; 23.
- Chukanova E.I. The influence of Cerebrolysin on the clinical manifestations and course of dyscirculatory encephalopathy. Journal of neurol and psychiat 2005; 1:42-45.
- Lombardi VR, Windisch M., Garcia M., Cacabelos R. Effects of Ccrcbrolysin on in vitro primary microglial and astrocytc rat cell cultures. Methods Find Exp Clin Pharmacol 1999; 21:5:331-338;
- Alvarez XA, Lombardi V.R., Fernandez-Novoa L. etal. Ccrcbrolysin reduces microglial activation in vivo and in vitro: a potential mechanism of ncuro-protcction. J Neural Transm Suppl 2000; 59: 281-292.
- Hartbauer M., Hutter-Paier V., Skofitsch G., Windisch M. Antiapoptotic effects of the peptidic drug ccrcbrolysin on primary cultures of embryonic chick cortical neurons. J Neural Transm 2001; 108:4:459-473.
- Gutman V., Hutter-Paier V., Skofitsch G et al. In vitro models of brain ischemia: the peptidcrgic drug Ccrcbrolysin protects chick cortical neurons from cell death. Ncurotoxity Res 2002; 4:1:59-65.
- Schauer E., Wronski R., Patockova J. etal. Ncuroprotcction of ccrcbrolysin in tissue culture models of brain ischemia: post lesion application indicates a wide therapeutic window. J Neural Transm 2006; 113:7:855-868.
- Doppler E., Alvarez A., Cacabelos R. et al. Syncrgistic treatment effects with Ccrcbrolysin and donepczil: Results from a randomized, double-blind, multiccntcr trial to compare safety and efficacy of Ccrcbrolysin, donepczil and a combination treatment in patients with probable Alzheimer's disease. Alzheimer's and Dementia 2009; 5:4:Suppl 1:248-249.
- Suchanek-Frohlich H., Wunderlich E. Randomisicrtc Doppclblind-Placebo-Vcrglcich sstudi with mit cincm Amino-saurc-Pcptid-Extrakt. Dcr Prak-tischcArzt 1987; 41: 11: 1027-1034.
- RuetherE., Husmann R., KinzJerE. et al. A28-wcck, double-blind, placebo-controlled study with Ccrcbrolysin in patients with mild to moderate Alzheimer's disease. Int Clin Psychopharmacol 2001; 16:5:253-263.
- Ruether E., Ritter R., Apecechea M. et al. Sustained improvements in patients with dementia of Alzheimer's type (DAT) 6 months after termination of CCRCbrolysin therapy. J Neural Transm Suppl 2000; 107:7:815-829.
- Bae CY, Cho CY, ChoK. et al. A double-blind, placebo-controlled, multiccntcr study of Ccrcbrolysin for Alzheimer's disease J Am Gcriat Soc 2000; 48: 12: 1566-1571.
- Muresanu DF, Rainer M., Moessler H Improved global function and activities of daily living in patients with AD: a placebo-controlled clinical study with the ncurotrophic agent Ccrcbrolysin. J Neural Transm Suppl 2002; 62: 277-285.
- Alvarez XA, Cacabelos R., Laredo M. et al. A24-wcck, double-blind, placebo-controlled study of three doses of Ccrcbrolysin in patients with mild to moderate Alzheimer's disease. Eur J Ncurol 2006; 13:1:43-54.
- Wei ZH, He QB, Wang H. et al. Mcta-analysis: the efficacy of nootropic agent Ccrcbrolysin in the treatment of Alzheimer's disease. J Neural Transm 2007; 114:5:629-634.
- Gavrilova S.I., Fedorova Ya.B., Kolykhalov I.V. et al. The therapeutic potential of Cerebrolysin in the preventive therapy of Alzheimer's disease. Journal of neurol and psychiat 2008; 108: 8:24-28.
- Alvarez XA, Sampedro C, Cacabelos R. et al. Induced TNF-alpha and increased IGF-I levels in the scrum of Alzheimer's disease patients treated with the ncurotrophic agent Ccrcbrolysin. Int J Ncuropsychopharmacol 2009; 17:1-6.
- Rockenstein E., Mante M., Adame A., Crews L. etal. Effects of Ccrcbrolysin on neurogencsis in an APP transgenic model of Alzheimer's disease. Acta Ncuropathol 2007; 113:3:265-275.
- Alvarez-Buylla A., Herrera DG, Wichterle H. The subvcntricular zone: source of ncuronal precursors for brain repair. Prog Brain Res 2000; 127:1-11.
- Jin K., Minami M., Lan JQ et al. Neurogencsis in dentate subgranular zone and rostral subvcntricular zone after focal cerebral ischemia in the rat. Proc NatlAcad Sci USA 2001; 4710-4715.
- Arvidsson A., Collin T., Kirik D. et al. Ncuronal replacement from endogenous precursors in the adult brain after stroke. Nat Mcd 2002; 8: 963-970.