Statistical method. Statistical methods were used to identify and evaluate risk factors for the following prognoses: annual mean aneurysm growth, complication rates (acute dissection and/or rupture), mortality, and long-term survival.
Survival analysis. Five-year survival estimates were calculated by life table analysis (Kaplan-Meier). Differences in survival were tested using the LIFEREG procedure in SAS version 6.07, 1994 (SAS Institute, Inc, Kari NS).
Kaplan-Meier survival plot. Five-year survival estimates are illustrated for patients with thoracic aortic aneurysms (TAAs) versus the general (age- and sex-matched) population (EA).
Results and discussion. Joyce JW et al. [21] reported a 5-year survival rate of 61% for ascending thoracic aortic aneurysms with a diameter of 6 cm or less; aneurysms larger than 6 cm had a five-year survival rate of 38% of cases. Complete five-year survival in our series was observed in 64% of cases. Mortality is expected to be related to the aneurysm in the vast majority of cases, although adequate details for this distinction have often been unavailable. Survival was significantly lower for descending aortic aneurysms (39% at 5 years) (p=0.031). Patients with dissection had lower survival (46% at 5 years).
Growth parameters. The estimated annual growth rate for thoracic aortic aneurysms was 0.29 cm/year. This is in close correlation with the results reported by Dapunt OE et al. [26] (0.32 cm/year). Masuda Y. et al. [27] reported a growth rate for thoracic aortic aneurysms of 0.13 cm/year. Hirose Y. et al. [28] report a high annual growth rate of 0.42 cm/year for thoracic aortic aneurysms. In a subsequent study, however, Hirose Y. et al. [28] gave a significantly lower rate.
The discrepancy between the two studies [28] may well explain the different strategies used to estimate growth. In recent studies by Hirose Y. [28], the regressions used are close to those used by Dapunt OE et al. [26] and in the current study, when assessing the growth of a thoracic aortic aneurysm. Earlier studies by Hirose Y.'s group [28] calculated that height is equal to the difference between the last and first measured size divided by the duration between studies.
Size has traditionally been considered an important risk factor for complications (ie, acute dissection and/or rupture) in patients with thoracic aneurysms, and it has been considered the most important independent factor in the decision to undergo elective surgery. The effect of size on the rate of growth of aneurysms is a matter of debate. Dapunt OE et al. [26] note that more intense dilations were found in those patients whose aortic diameter at diagnosis was >5 cm. On the other hand. Hirose Y. et al. [28] found no significant effect of size on growth rate.
Dapunt OE et al. [26] reported that the presence of hypertension correlates with a large aortic diameter, but does not significantly affect the rate of aortic enlargement growth. Masuda Y. et al. [27] reported a direct relationship between the magnitude of diastolic pressure and the degree of aortic dilatation.
Possibility of complications. It is important to consider the natural history of patients with thoracic aneurysms, e.g. cases of acute dissection and/or rupture in this population. Pressler V., McNamara JJ [22] reported that in eight of nine cases of ruptured descending thoracic aortic aneurysms, the aortic size was greater than 10 cm. Subsequent studies reported complications with much smaller sizes. A study by Gott VL et al [11] of ascending aortic aneurysms in patients with Marfan syndrome reported a mean size of 7.8 cm at the time of dissection.
However, in seven of 26 patients (26.9%), dissections were observed with aneurysms measuring 6.5 cm or smaller. In addition, Crawford ES et al. [9] reported a mean size at rupture of 8.0 cm among 117 patients with descending thoracic and thoracoabdominal aneurysms. Dapunt OB et al. [26] report ruptures of thoracic aneurysms occurring in even smaller sizes, with an average size of 6.1 cm.
These observations indicate dissections or ruptures as aneurysms expand in size. The average size at the time of rupture or dissection was 6.0 cm for ascending aneurysms and 7.2 cm for descending aneurysms. A multivariate retrospective analysis was performed to isolate risk factors for acute dissection or rupture, which showed that a size greater than 6.0 cm increased the likelihood of dissection or rupture by 32.1% for ascending aneurysms (p=0.005). For descending aneurysms, this probability increased by 43% for sizes greater than 7.0 cm (p=0.006). Frist WH et al. [33] provide the following data for ascending and descending aneurysms: 6 cm for the ascending aorta and 7 cm for the descending aorta.
Criteria for surgical intervention.
Below we have attempted to demonstrate the importance of the thoracic size of the aneurysm in dire complications such as aortic rupture and dissection. Our observations attempt to demonstrate a clearly increasing incidence of complications with increasing aortic size. These observations indicate that the average size at rupture and dissection is 6.0 cm for ascending aneurysms and 7.2 cm for descending aneurysms. Multivariate analysis of risk factors affecting acute dissection and/or rupture shows that size greater than 6.0 cm is a significant risk factor (p=0.005). Logistic analysis indicated a 32.1% increase in the likelihood of dissection or rupture for ascending aneurysms with a mean size greater than 6.0 cm (p=0.005) and a 43.0% increase for descending aneurysms greater than 7.0 cm (p=0.006).
These data strongly call for the use of a size criterion for surgical replacement of aneurysmal aorta to prevent complications of rupture and dissection. Additionally, these data suggest that a lower size criterion than previously recommended should be used.
If the average size at the time of complication (in this case: 6.0 cm for the ascending and 7.2 cm for the descending aorta) is used as an intervention criterion, then half of the patients are likely to experience serious complications during the intervention. Accordingly, the authors suggest intervention for slightly below average size criteria at the time of complication. It is suggested that surgical intervention be used for 5.5cm ascending and 6.5cm descending aortic aneurysms. These above criteria allow intervention to occur before the catastrophically increased likelihood of rupture and/or delamination.
These recommendations indicate that elective surgery is much safer than emergency surgery. For ascending aneurysms and aortic arch aneurysms, elective surgery was very safe (mortality rate 4.3%). These mortality results are broadly consistent with those reported by other centers. Although surgery on the descending aorta has a higher risk (19%), the number of patients in this category is relatively smaller to influence the final outcome.
Many of the above data have shown that surgery on the ascending aorta is safer. CPB via left atriofemoral cannulation using a centrifugal pump has been shown to be a safe and reliable method for preventing postoperative complications such as ischemic complications such as renal failure and spinal cord injury. In addition, the advent of a protease inhibitor (aprotinin) led to a significant reduction in blood loss as a result of the protection of platelet wall receptors at the beginning of CPB. The introduction of a collagen-impregnated Dacron graft has led (Hemashield Meadox Medicals Inc, Oakland, NJ) to improve surgical hemostasis, essentially eliminating bleeding through the graft wall. These facts support priority selective intervention to avoid rupture and dissection of aneurysms.
For descending aortic aneurysms, the risk of paraplegia should be considered as a significant intraoperative complication. The risk of paraplegia (spinal origin) in the literature remains between 2 and 20%, in this case it is 4.0%. Obviously, advanced age and concomitant diseases can lead to frequent complications for which surgical intervention is not indicated. Thus, each patient must be assessed individually, and the expected risk of complications (especially paraplegia in descending aortic aneurysms) must be weighed to avoid complications such as rupture and dissection. In addition, the level of doctors in the medical center in question must be taken into account. The size criteria presented here are suggested for less severely ill patients treated at more experienced centers.
Studies of patients with Marfan syndrome.
More than 90% of deaths in Marfan syndrome are due to complications of ascending aortic aneurysms. Because most patients with Marfan syndrome have some degree of aortic regurgitation (aortic root 6.0 cm), Gott, Lima et al recommend prophylactic treatment for aneurysms that reach 5.5 to 6.0 cm. We recommend an intervention criterion of 5.0 cm for patients with Marfan syndrome, other hereditary collagen vascular diseases, or familial cases of aortic regurgitation.
This size criterion is slightly lower than the recommendations for intervention for atherosclerotic aneurysms of the ascending aorta. Experience has shown that in several patients with Marfan syndrome the dissection or rupture occurred at sizes less than 5.0 cm. These young patients were often in severe clinical condition. Thus, prophylactic surgical treatment can significantly improve their clinical condition and prognosis.
Developing intervention criteria is a complex research endeavor. Research into this problem is important to improve patient survival. To determine appropriate size criteria for the intervention, we used statistical analysis. These intervention criteria should take into account the patient's age, physical condition, life expectancy, that is, approach the intervention criteria using statistical methods in terms of preventing complications (dissection and rupture). Organ pressure from the aneurysm, concomitant aortic regurgitation, and acute aortic dissection are widely accepted common indications for surgical intervention regardless of aortic size.
Some points of view are still debated in the surgery of chronic ascending aortic disease: which technique to use in treatment (open or wrap technique, direct or indirect reimplantation of the coronary arteries), how to protect the brain during the restoration of the aortic arch. When the aortic root was included in the surgical plan, implantation of a valved conduit was typically performed using a wrap technique with coronary artery reimplantation and a Dacron graft (8mm). Valve replacement alone does not protect against recurrence of ascending aortic aneurysm, leading to reoperation. However, repeated operations have a high mortality rate.
Some authors have used the wrapper technique. Regarding bleeding, the probability of early reoperation is low - 4.5% in this series and can be compared with the data of Kouchoukos N. (2% - open technique). In the studied patients there was no need for repeated operations for pseudoaneurysms of the ascending aorta.
Coronary reimplantation using a Dacron graft (8mm) appears to be more reliable and technically feasible than direct reimplantation or reimplantation with aortic buttons. Kouchoukos N. [7] Taniguchi K. et al. [12] reported the appearance of pseudoaneurysms in the coronary ostium after these operations [12,13].
It is therefore believed that the use of a Dacron graft prevents these complications (as shown by standard coronary angiography). The resulting right atrial fistulas closed, with the exception of one case where reoperation was required. Nine cases in which a tubular graft was implanted resulted in sudden death of unknown cause (no autopsy was performed). The possibility of false aneurysms or fistulas leading to death was not excluded.
Aortic arch repair is always indicated when the aneurysm extends into the aortic arch, when the dissection extends into the arch, or when the intimal tear is localized to the arch (2 cases in this series). The authors believe that stopping hypothermic CPB leads to neurological complications. Griepp RB et al. reported a rate (5.6%) of neurological complications in 87 patients [15]. The authors prefer stopping hypothermic CPB during selective catheterization of the great vessels of the brain.
30-day mortality was observed in 7.6% of patients and 6% in patients with prosthetics (valve-containing conduits were used). Galloway AC et al. [6] reported similar case fatality rates of 5.3 and 5%, respectively.
Many analyzes also indicate that concomitant coronary artery transplantation and older age are major risk factors, as has been shown by other authors. Survival is poor in patients undergoing surgery for chronic ascending aortic disease: 48% at 12 years in Kouchoukos NT et al. [13] and 57% over 7 years in Culliford AT [34]. In this series, complete survival was 59.6% ± 3.7% over 9 years for all patients and 66.3% ± 4.5% over 9 years for patients with prosthetics with a valve-containing conduit (intraoperative mortality was also included).
The predominant cause of late mortality was not found in this series. Factors that increase risk as shown by univariate analysis are: CPB time, aortic arch repair, chronic aortic dissection, and older age.
Conclusion
Medical Internet conferences
Introduction
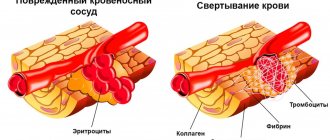
Atherosclerosis and aortic aneurysms have common risk factors, but the development of an aneurysm is associated with the strength and elastic properties of the aortic vascular wall. An aortic aneurysm is a permanent local or diffuse expansion of its lumen by 2 times or more compared with an unchanged area or the normal diameter of the aorta. The first descriptions of an abdominal aortic aneurysm date back to the 16th century. (Vesalius), and Laennek was the first to describe the symptoms of a ruptured abdominal aortic aneurysm. In 1955, Etheredge performed the first successful operation for a thoracoabdominal aneurysm.
Among aneurysms of all locations, abdominal aortic aneurysms account for 29-37.8%. The ratio of the incidence of this disease in men and women is 5:1. In 95-96% of patients, aneurysms are located below the origin of the renal arteries. The main etiological factor in the development of aneurysms of the abdominal aorta is atherosclerosis (42-73%) [1, 3].
Atherosclerosis and aortic aneurysms have common risk factors, but the development of an aneurysm is associated with the strength and elastic properties of the aortic vascular wall. With an aneurysm in the aortic wall, there is a decrease in elastin content and an increase in collagen, which leads to a loss of strength of the aortic wall, and its subsequent expansion accelerates the development of the atherosclerotic process. According to Laplace's law, an increase in vessel diameter increases wall stress, and aneurysm formation accelerates, and in individuals with an aortic diameter greater than 50 mm, the increase in aneurysm size is 4.3 mm per year.
The development of modern research methods is accompanied by the widespread introduction of computer modeling in surgery in various fields, in particular cardiovascular surgery. Knowledge of the deformation and strength properties of the tissues of the arterial vascular wall, as well as the variability of these properties with age, is necessary for computer modeling to predict the development of possible complications during surgical reconstructive interventions [2, 4].
Materials and methods
The material for the study was: 5 unfixed aortas with dissecting abdominal aneurysm and 10 aortas without macroscopic signs of pathology, removed from the corpses of males (n 15) aged 35-60 years no later than the first day after death. The outer and inner diameters, wall thickness, extent and localization of aneurysmal expansion were studied. Measurements of the outer and inner diameter of the abdominal aorta were carried out using the Ioffe method using digital calipers, with a step of 0.5 mm.
results
The mean age of the study subjects was 53±5 years. The study revealed that the outer diameter of the aortas in the presence of aneurysms was 61.4±0.7 mm, the inner diameter was 45.5±0.6 mm, while the wall thickness reached 15.5±0.5 mm. In a study of 10 aortas without macroscopic signs of pathology, the following average values were obtained: at the level of the origin of the celiac trunk (No. 1), the thickness of the aortic walls averaged 2.9 ± 0.3 mm, the outer diameter was 19.6 ± 0.5 mm, internal diameter – 16.7±0.5 mm. At the level of the origin of the renal arteries (No. 2): the thickness of the aortic walls was 3.1±0.4 mm, the outer diameter was 17.5±0.7 mm, the inner diameter was 14.4±0.6 mm. 3 cm below the level of the origin of the renal arteries (No. 4) with an average thickness of the aortic walls of 3.2±0.4 mm, the outer diameter was 15.5±0.6 mm, the inner diameter was 11.4± 0.4 mm.
A comparative analysis revealed: the thickness of the walls of the aortic aneurysm affected exceeded the values of the aortas not affected by the aneurysm: at the level of the origin of the celiac trunk (No. 1), at the level of the origin of the renal arteries (No. 2), in the area of the aneurysm (No. 3) and 3 cm below the level of the origin renal arteries (No. 4) by 1.7: 1.8: 4.1 and 1.6 times, respectively. There was also an increase in the outer diameter at the above levels: 1.1: 1.3: 3.3 times, respectively, but at level No. 4 there was a sharp decrease - 2.5 times. The internal diameter of the aorta affected by the aneurysm exceeded the internal diameter of the unaffected aortas, so its increase at levels No. 2 and No. 3 was 1.2 and 3.2 times, respectively. At levels No. 1 and No. 4, a narrowing of the internal lumen was observed, a decrease in the internal diameter was 1.4 and 14 times.
When conducting a full-scale experiment, it was revealed that the Young's modulus of aortic tissue without pathology was 12 MPa, in the presence of an aneurysm (in the zone of maximum dilatation of the aneurysm) - 3.2 MPa, and in case of damage to the aortic wall by atherosclerosis - from 20 to 31 MPa.
Discussions
The development of abdominal aortic aneurysms is characterized by changes in the morphological, strength and elastic-deformable properties of its wall. The nature of the decrease in these properties has a direct relationship with the intensity and localization of atherosclerotic lesions in the aneurysmally altered wall of the abdominal aorta, and its subsequent expansion accelerates the development of atherosclerosis, which aggravates the course of both pathological processes.
conclusions
Aneurysmal and atherosclerotic lesions are among the most common pathological processes of the vascular wall, in particular the wall of the abdominal aorta. Understanding their combined development makes it possible to determine changes in the biomechanical characteristics of the aorta, and also makes it possible to predict the tactics of surgical treatment and the risks of possible complications.
Ultrasound of the heart: norm and interpretation of results
In various fields of medicine, ultrasound examination of internal organs is the main diagnostic method.
Ultrasound of the heart in cardiology is called echocardiography . The study allows us to identify changes, anomalies, disorders and malformations in the functioning of the heart. The procedure is painless, non-invasive, and can be prescribed to people of all ages and even pregnant women. Ultrasound examination of the heart is carried out already at the stage of intrauterine development of the fetus.
For the examination, special equipment is used - an ultrasound machine or scanner. A special gel is applied to the patient’s skin, which promotes better penetration of ultrasound into the heart muscles and other structures, and a sensor is attached. The data is displayed on the monitor and automatically recorded.
The procedure lasts from 30 to 60 minutes. Echocardiography is carried out by a cardiologist, as well as in the direction of a pulmonologist, neurologist, endocrinologist, and gynecologist.
The doctor prescribes an examination if the patient has complaints of dizziness, shortness of breath, chest pain, weakness, arrhythmia, tachycardia, high blood pressure, signs of heart failure, and fainting. People who have had cardiac surgery or a heart attack need to undergo the procedure once a year.
Echocardiography is used for:
- determining murmurs, - diagnosing the condition of valves, - detecting changes in structures, - assessing the functioning of parts of the heart in people with chronic diseases, - detecting fluid accumulation, - assessing and monitoring birth defects, blood flow, the condition of blood vessels, - detecting blood clots in the chambers
Ultrasound of the heart shows the condition of the pericardium, myocardium, blood vessels, mitral valve, and ventricular walls.
During the procedure, the cardiologist records the readings obtained. Decoding the data makes it possible to identify diseases, abnormalities, pathologies, and anomalies in the functioning of the heart. Based on the information received, the doctor makes a diagnosis and prescribes treatment. Often, in addition to echocardiography, Doppler ultrasound is prescribed. This procedure allows you to see the direction of movement, determine the speed and turbulence of blood flow in the chambers of the heart.
The doctor makes a conclusion in the diagnostic results report, which displays the data obtained from the ultrasound machine.
Echocardiography is used to diagnose:
— pre-infarction state; - heart failure; - ischemic disease; - myocardial infarction; - hypertension, hypotension; - heart defects; - rhythm disturbances; - cardiomyopathy; - myocarditis, pericarditis; — vegetative-vascular dystonia; - rheumatism.
In order to obtain data on the work of the heart during physical activity, stress echocardiography is performed. The patient is given a certain physical activity or the heart muscle is stimulated to work harder with the help of drugs, and readings are taken.
Quite rarely in practice there are cases when it is not possible to carry out a standard ultrasound procedure of the heart: deformation of the chest, the presence of prosthetic valves, a layer of subcutaneous fat, abundant hair growth. In this case, transesophageal (transesophageal) echocardiography is performed.
Types of echocardiography:
- One-dimensional in M-mode. The sensor produces waves along one selected axis. An image is displayed on the monitor - a top view. Makes it possible to see the aorta, atrium, and ventricles.
- Two-dimensional. Makes it possible to obtain an image in two planes and analyze the movement of structures.
Only a cardiologist can conduct a complete and accurate analysis of the cardiogram transcript and draw a conclusion.
Heart ultrasound standards:
Left ventricle
- myocardial mass (men: 135-182 g; women: 95-141 g)
— myocardial mass index (men: 71-94 g/m2; women: 71-80 g/m2)
- volume at rest (men: 65-193 ml; women: 59-136 ml)
- resting size (men: 5.7 cm; women: 4.6 cm)
- size during contraction (men: 4.3 cm; women: 3.1 cm)
— wall thickness outside of heart contractions during work: 1.1 cm. An indicator of 1.6 cm indicates hypertrophy
— ejection fraction of at least 55-60%. The indicator indicates the volume of blood that the heart ejects with each contraction. A lower value indicates heart failure
— stroke volume: 60-100 ml (the amount of blood ejected per contraction)
Right ventricle
— wall thickness 5 mm
— size index 0.75 — 1.25 cm/m2
- size at rest 0.75 - 1.1 cm
Valves
— A decrease in the diameter of the valve opening, difficulty pumping blood, indicates stenosis
— Heart failure is diagnosed if the valve flaps prevent the reverse flow of blood and do not perform their intended function
Pericardium
— Liquid norm is 10-30 ml. When the reading is over 500, normal heart function is difficult. The onset of an inflammatory process is possible - pericarditis, accumulation of fluid, formation of adhesions of the heart and pericardial sac.
Carrying out a cardiac ultrasound procedure helps to detect diseases of the cardiovascular system at an early stage and take the necessary measures in time.