In case of ventricular tachycardia, the patient should be immediately provided with competent assistance to avoid death. The sequence of actions is described below, as well as the necessary medications that will make a person feel better.
- Tachycardia with wide QRS complex
- Treatment
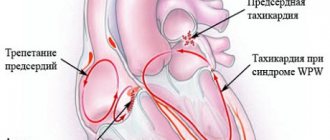
- WPW and PQ phenomena
- Risk of atrial fibrillation and flutter
Cardiopulmonary resuscitation (CPR) before connecting the ECG monitor.
- If ventricular fibrillation/ventricular tachycardia (VF/VT) is present, the monitor shows three consecutive defibrillator shocks with gradually increasing strength.
- If VF/VT persists or recurs, continue CPR, tracheal intubation, and access to the vein.
- Adrenaline 1 mg every 3-5 minutes.
- Repeated defibrillator shocks.
- Cordarone 5 mg/kg intravenously (or lidocaine 1.5 mg/kg) for 3-5 minutes if there is no effect after the 3rd discharge of the defibrillator.
What causes ventricular tachycardia?
What causes ventricular tachycardia?
Ventricular tachycardia most often occurs in patients with organic changes in the heart, for example, myocardial infarction, cardiomyopathy, myocarditis. Scar tissue creates abnormal electrical pathways, causing the heart to beat at a high rate. Sometimes people without heart disease can develop ventricular tachycardia. It is easier to treat and is usually not life-threatening.
Diagnostics
Photo: sante.by
A cardiologist examines patients with cardiac arrhythmias. Diagnostics is carried out comprehensively and includes the following methods:
- Collection of complaints and anamnesis data. Most patients who consult a doctor have characteristic symptoms of tachycardia: rapid heartbeat, chest pain, dizziness. The majority have a confirmed cardiac pathology, for example, a history of myocardial infarction.
- Physical examination. The palpation method determines the increase in heart rate from 100 to 220 beats per minute. The rhythm is often correct. A decrease in blood pressure is also characteristic.
- Laboratory methods. The results of a biochemical blood test indicate a decrease in the level of electrolytes: potassium, magnesium, calcium. For patients over 40 years of age, blood lipid levels and a coagulogram are recommended.
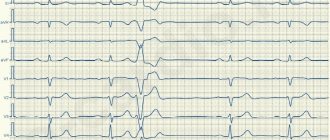
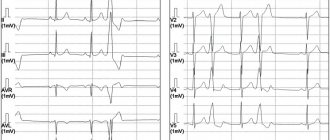
- Instrumental diagnostics. Gastric tachycardia is confirmed by electrocardiogram data. The chart has many features that can only be deciphered by a doctor. The most common features: wide QRS complexes (more than 0.12 seconds), dissociation of P waves and supraventricular “captures”, uniformity of QRS vectors (chest leads). Additional research methods are daily Holter ECG monitoring, exercise tests, ECHO-CG.
What are the symptoms of ventricular tachycardia?
What are the symptoms of ventricular tachycardia?
Due to the fact that during ventricular tachycardia, the contractions of the ventricles are not synchronized with the atria, a chaotic heartbeat occurs, which prevents sufficient filling of the heart chambers with blood, as a result of which the heart is not able to pump the required amount of blood to vital organs. This may lead to loss of consciousness. However, the clinical manifestations of ventricular tachycardia may vary. Sometimes short episodes of ventricular tachycardia may last seconds and not cause any noticeable symptoms. In other cases, a prolonged episode (usually more than 30 seconds) can lead to severe symptoms such as:
Palpitations Dizziness Shortness of breath Chest pain Loss of consciousness
Ventricular tachycardia sometimes poses a serious danger, leading to a more severe, life-threatening condition - ventricular fibrillation. This is a condition in which the ventricles of the heart seem to tremble and pump very little blood. Ventricular fibrillation is the most common cause of sudden cardiac death.
WPW and PQ phenomena
In such cases we are talking about the WPW phenomenon (Kent's beam) or the phenomenon of the shortened PQ interval (James' beam).
Main ECG criteria for the WPW phenomenon:
- shortening of the R-L interval (less than 0.12 s);
- the presence of a delta wave at the initial rise of the QRS complex;
- widening of the QRS complex due to the presence of a delta wave;
- change in the final part of the ventricular complex.
It should be noted that the physician’s ignorance of the ECG signs of the WPW phenomenon is the reason for the incorrect interpretation of these changes as signs:
- acute myocardial infarction,
- hypertrophy of both ventricles
- or blockade of the legs of the atrioventricular bundle.
The WPW phenomenon, as a rule, does not require treatment.
ECG signs of preexcitation syndrome against the background of sinus rhythm vary widely, which is associated with the degree of preexcitation and the constancy of conduction along the AP. The following options are possible:
1) the ECG constantly shows signs of pre-excitation (manifest pre-excitation syndrome);
2) on the ECG, signs of preexcitation are transient (intermittent or transient preexcitation syndrome);
3) The ECG is normal under normal conditions, signs of pre-excitation appear only during the period of paroxysm (latent pre-excitation syndrome).
The diagnosis of WPW syndrome is established in the presence of a combination of ECG signs of ventricular preexcitation with paroxysms of tachyarrhythmia.
In the majority of patients (90%), doctors record this type of atrioventricular reciprocal tachycardia, in which the excitation wave propagates anterogradely through the atrioventricular node to the ventricles and retrogradely through the accessory tract to the atrium. This tachycardia is called orthodromic.
Much less often (5-10%) one can observe a variant of atrioventricular reciprocal tachycardia, in which the excitation wave makes a circular motion along the same loop, but in the opposite direction: anterograde along the AP to the ventricles and retrograde through the atrioventricular node to the atrium - this tachycardia is called antidromic.
The ECG records a paroxysm with extended f.i.8 complexes, more than 0.1 s (like the most pronounced preexcitation, “ventricular type”) with a frequency of 150-200 per minute.
How is ventricular tachycardia diagnosed?
How is ventricular tachycardia diagnosed?
Ventricular tachycardia can be diagnosed using an electrocardiogram at the time of the attack. For long-term recording of an electrocardiogram if ventricular tachycardia is suspected, a Holter monitor is used - continuous recording of an ECG over time. Additional tests such as echocardiography, cardiac MRI, stress test, or coronary angiography may be needed to determine the presence of structural heart disease.
Medicines
Photo: zagranmaster.ru
The main group of medications used to treat ventricular tachycardia are antiarrhythmic drugs. Theoretically, class I, II and III drugs can be prescribed. However, extensive practical experience has made it possible to divide them into three orders of decreasing efficiency. First-line drugs include drugs that give a noticeable positive result in 70% of cases. An example of such a drug is Amiodarone. However, the list is not limited to them; the selection of the safest and most effective medicine is made by a doctor.
Self-treatment with antiarrhythmic drugs is strictly prohibited, as they can provoke adverse reactions, including death. Even for the safest drugs in this group, the frequency of undesirable effects ranges from 4-8% to 25-30%. It is worth starting treatment under constant monitoring of heart rate and under the supervision of a doctor.
How to treat ventricular tachycardia?
It is very important to identify the cause of ventricular tachycardia for the most effective treatment.
The main methods of treating ventricular tachycardia are as follows:
| Cardioversion | Antiarrhythmics | Cardioverter-defibrillator | Radiofrequency catheter ablation |
| Sustained ventricular tachycardia often requires urgent treatment. Electrical cardioversion is a procedure in which an external defibrillator and electrical current are used to restore normal heart rhythm. | These drugs, when taken long-term, can prevent new attacks of arrhythmia. Antiarrhythmics can also be used to urgently restore the correct rhythm, then they are administered intravenously. | Patients at high risk of developing ventricular tachycardia are implanted with a cardioverter defibrillator. This device is implanted under the skin of the chest and, when an arrhythmia occurs, automatically sends an electrical shock to the heart, causing the heart to return to a normal rhythm. | This procedure is used to destroy small areas of heart tissue responsible for causing arrhythmias. Catheter ablation may reduce the number of episodes or completely eliminate ventricular tachycardia. In patients without myocardial pathology, ventricular tachycardia is successfully treated with catheter ablation and is first-line therapy. Modern technologies allow the surgeon to accurately identify the pathological path of the arrhythmia and eliminate abnormal signals that contribute to the occurrence of tachycardia. The procedure for ablation of ventricular tachycardia for structural heart disease can be longer and more complex. |
Average reading time: 3 minutes .
Yu.A. Bunin RMAPO, Moscow
Ventricular heart rhythm disturbances (VHD) and, first of all, ventricular tachycardia, most often associated with ischemic heart disease and its complications or hereditary genetic pathology, seem to be the most important risk factors for the development of sudden cardiac death (SCD). Thus, the cause of SCD in developed countries in 75-80% of cases is ventricular fibrillation (VF) and bradyarrhythmias play a much lesser role in its development. At the same time, in a number of cases, VNRS in patients without organic heart disease and without genetic diseases (long or short QT interval syndrome, Brugada syndrome, arrhythmogenic right ventricular cardiomyopathy, etc.) probably do not have a significant negative impact on the prognosis of life.
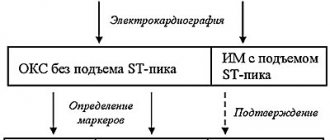
Electrocardiographic, electrophysiological diagnostics and clinical manifestations of ventricular tachycardia On an ECG, ventricular tachycardia (VT) is diagnosed in the presence of three or more consecutive premature ventricular complexes, the width of which, as a rule, exceeds 0.12 sec (usually 0.14 sec or more) . Rarely, QRS complexes during VT can be narrow (0.12 sec or less), in particular, when the place of impulse formation is in close proximity to the bifurcation of the His bundle and the anterograde impulse spreads to the ventricles along its branches. There are monomorphic (the shape of the QRS complexes does not change during tachycardia) and polymorphic VT, in which the ventricular complexes change their morphology. A special type of polymorphic VT that occurs in patients with a prolonged QT interval is called tachycardia of the “pirouette” type, or “torsades de pointes”. Like supraventricular tachyarrhythmias, VT can be paroxysmal or non-paroxysmal (chronic). If the tachycardia continues for more than 30 seconds, it is classified as sustained VT, and if it stops spontaneously in less than 30 seconds, it is called unsustained. VT must be differentiated from supraventricular tachycardia (SVT) with aberration (unusual conduction of impulses through the ventricles), since both of these rhythm disturbances have the appearance of tachycardia with regular wide QRS complexes. The causes of the aberration are blockade of the His bundle branch, preceding tachycardia or tachycardia-dependent during it, and conduction of the supraventricular impulse along the additional extranodal atrioventricular tract (APP). The clinical significance of differential diagnosis is not in doubt due to the differences in prognosis and treatment approaches between SVT and VT. It is usually carried out on the basis of an analysis of a surface ECG, but in difficult cases it is necessary to resort to intracardiac EPI. It should be noted that VT is the most common cause of tachycardia with wide QRS complexes. The most important electrocardiographic criteria for diagnosing VT are listed below. 1. Presence of signs of atrioventricular (AV) dissociation: location of P waves independent of QRS complexes, registration of “captured” and/or “drain” ventricular complexes. When retrograde conduction of ventricular impulses to the atria is maintained, the P waves following the QRS complexes are inverted in leads II, III, AVF. 2. The width of the ventricular complexes is more than 0.14 seconds (QRS complexes, as with blockade of the right branch of the His bundle) or more than 0.16 seconds (QRS complexes, as with blockade of the left branch of the His bundle). These signs do not help in differential diagnosis when there was already a blockade of the His bundle branch in sinus rhythm, and in cases of propagation of the supraventricular impulse along the accessory pathway or previous use of class 1C antiarrhythmics: in patients with SVT, the duration of the ventricular complex may be more than 0.14 sec ( tachycardia “BVP-type”) and more than 0.16 seconds (tachycardia “BVP-type”). 3. The distance from the beginning of the R wave to the nadir (the lowest point of the S wave) exceeds 0.1 seconds in one of the chest leads. 4. Concordant QRS complexes in all chest leads (positive or negative). However, positive concordant ventricular complexes can be observed with antidromic AVRT (localization of the AP in the posterior wall on the left). 5. If the ventricular complexes resemble a block of the right branch of the His bundle: monophasic or biphasic QRS complexes in leads V1 and RS (R 6. If the ventricular complexes resemble a block of the left branch of the His bundle: the width of the r wave in leads V1 and V2 exceeds 0.03 sec, and the distance from the beginning of the ventricular complex to the nadir of the S wave exceeds 0.06 sec. In lead V6 there may be a small q wave with a large R wave or a ventricular complex in the form of QS. 7. In the presence of blockade of the His bundle branch in sinus rhythm, tachycardia with wide with QRS complexes that differ from those in sinus rhythm, most likely ventricular. Electrophysiologically, VT is characterized by the presence of a short or “negative” (i.e., recorded after the ventricular complex) HV interval or even the absence of a His bundle potential (coinciding with the ventricular complex). stimulation tachycardia in the configuration of QRS complexes is usually similar to spontaneous.Long-term (Holter) ECG monitoring provides additional important information about the quantitative and qualitative characteristics of episodes of symptomatic and asymptomatic (usually unstable) VT, which is important for determining tactics for managing patients and identifying high-risk groups by sudden death. Speaking about the diagnosis of VT, it is necessary to note the certain importance of signal-averaged electrocardiography, which records late ventricular potentials, which reflect foci of slow myocardial conduction, which are the substrate for the development of arrhythmias by the re-entry mechanism. Up to 85% of patients with sustained VT have a positive (registration of late potentials) signal-averaged electrocardiogram. In post-MI patients with a negative signal-averaged ECG and normal contractile function of the left ventricle, the development of ventricular arrhythmias seems unlikely. At the same time, registration of late ventricular potentials in patients with post-infarction cardiosclerosis and an ejection fraction of less than 40% indicates a 30-40% risk of dangerous ventricular arrhythmias or sudden death [2]. In more than 60% of patients with sustained monomorphic ventricular tachycardia, its cause is coronary heart disease. Moreover, a significant proportion of them have a history of myocardial infarction (MI), often complicated by severe left ventricular systolic dysfunction. About 75% of patients resuscitated due to cardiac arrest had coronary artery disease. The occurrence of sustained VT later than 48 hours from the onset of MI is associated with a high risk of arrhythmia recurrence and increased mortality. If antiarrhythmic therapy is not administered, the 1-year mortality rate in these patients ranges from 50 to 70%. On the other hand, an accelerated idioventricular rhythm that appears in the early stages of acute myocardial infarction does not worsen the prognosis. A less significant group of VT is represented by patients with dilated and hypertrophic cardiomyopathies, acquired valvular and some congenital heart defects, congenital prolongation or shortening of the QT interval, arrhythmogenic dysplasia of the right ventricle, Brugada syndrome. There is also idiopathic VT (primary electrical heart disease), in which the prognosis is good. The average age of patients with VT exceeds 50 years, and 2/3 of them are male. For a long time, the most cited classification of ventricular arrhythmias (VA) was the classification of V. Lown, in which they were divided into five gradations, and this division was based primarily on their electrocardiographic quantitative and morphological characteristics. However, as is known, the degree of risk of developing fatal VA, and therefore the prognosis of patients, depends not only on the rhythm disturbance itself, but also on other factors, including the functional state of the myocardium, coronary blood flow, the state of the autonomic innervation of the heart, etc. In connection with the above The clinical classification of JT Bigger [3] is of great practical importance, although it now does not seem complete enough. Unlike V. Lown's classification, it includes not only a quantitative assessment of ventricular arrhythmias, but also an analysis of their clinical manifestations, as well as structural myocardial lesions. In the JT Bigger classification, all VAs are divided into three groups: benign, potentially malignant and malignant. Benign VAs currently include ventricular extrasystole of any grade, occurring without hemodynamic disturbances, in patients without organic heart disease. The prognosis for these patients is good, and there are no indications for antiarrhythmic therapy. The exception is when it is necessary to improve the quality of life with poor subjective tolerability of arrhythmias. An important difference between potentially malignant ventricular arrhythmias and benign ones is the presence of organic heart disease, especially with signs of left ventricular dysfunction. The largest group among them consists of patients who have had an MI. These individuals have an increased risk (according to JT Bigger - “average risk”) of sudden death due to the development of ventricular fibrillation. About 20% of patients with post-infarction cardiosclerosis have potentially malignant ventricular arrhythmias. In patients who have had an MI, in the presence of ventricular extrasystoles (more than 10 per hour), the risk of sudden death increases twofold and two to four times when nonsustained VT is recorded. The main goal of treating patients with potentially malignant VAs is to reduce mortality. To accomplish this, it is necessary to use antiarrhythmic drugs, the effectiveness of which (increasing survival) has been proven in large controlled studies. Attempts to use any antiarrhythmics to suppress ventricular ectopic activity when performing this task can, in some cases, lead to a worsening prognosis for life (Table 1). To illustrate this point, we can refer to the multicenter, randomized placebo-controlled studies CASTI and CASTII [4, 5], which studied the effect of antiarrhythmic therapy with class 1C drugs (flecainide, encainide, moricizine - an analogue of the domestic ethmosin) on the survival of patients with ventricular extrasystole, who have had an MI. Both studies were stopped early due to a significant and significant increase in mortality in patients taking antiarrhythmics compared with the placebo group. Other studies have shown that the use of antiarrhythmics of classes 1A and 1B (quinidine, disopyramide, mexiletine) in this category of patients is also associated with a risk of increased mortality [6]. However, a number of clinical studies and their meta-analysis have demonstrated that beta-blockers without their own sympathomimetic activity (propranolol, metoprolol, atenolol, timolol and some others), used in fairly high doses, reduce overall mortality by 20-25% in patients in the high-risk group. risk after a Q-wave MI. According to a meta-analysis of 13 randomized placebo-controlled trials, including 6553 patients with post-infarction cardiosclerosis and/or congestive heart failure who had potentially malignant VAs, amiodarone reduces overall mortality by 13% (p = 0 .03) and sudden death by 29% (p = 0.003) [7]. It should be noted that a detailed analysis of studies with amiodarone indicates that it reduces overall mortality mainly in patients with non-ischemic cardiomyopathy and non-sustained VT, combined with low ejection fraction (on average less than 40%). This was demonstrated quite clearly in the GESICA [8] and CHF-STAT [9] studies. The effectiveness of the combination of amiodarone with beta-blockers in patients after myocardial infarction was analyzed in two large studies, EMIAT (European myocardial infarct amiodaron trial) and CAMIAT (Canadian amiodaron myocardial infarction trial): cardiac death, as well as arrhythmic death plus resuscitation due to cardiac arrest were significantly less common in patients receiving b-blockers and amiodarone than when using only amiodarone or placebo [10]. Therefore, if amiodarone is planned for the treatment of symptomatic arrhythmia in a post-MI patient taking a beta-blocker, beta-blocker treatment should be continued. At the same time, in a double-blind, placebo-controlled study (1456 patients who had suffered an MI), although sotalol reduced overall mortality by 18%, these data were not statistically significant [14a]. The new class 3 antiarrhythmic dofetilide has no effect on the mortality of patients who have had an MI and patients with heart failure [14b]. Of particular interest is the MADIT study (Multicenter automatic defibrillator trial), which compared the effectiveness of implanted cardioverter defibrillators (ICDs) and antiarrhythmic drugs (80% of patients received amiodarone) in the primary prevention of death in patients with coronary artery disease after myocardial infarction with an EF less than 35% and unstable VT in which EPI induced sustained VT. The study was stopped early due to higher survival rates in patients with ICD [14]. Of great importance for the primary prevention of death are the results of the MADITII study, which demonstrated a 31% reduction in mortality in patients who had an MI and had an EF of 30% or less with an ICD, compared with a group of similar patients receiving traditional drug therapy (b-blockers, ACE inhibitors, etc.) [14c]. Thus, to improve the prognosis of life in patients who have suffered an MI and have potentially malignant VAs, the administration of b-blockers without their own sympathomimetic activity and, probably, amiodarone is indicated. Amiodarone appears to be the drug of choice for the treatment of symptomatic arrhythmias in patients with heart failure. Implantation of a cardioverter-defibrillator is recommended for patients whose EF remains less than 30% one month after myocardial infarction, regardless of the presence or absence of VNRS. It is inappropriate to use class 1 antiarrhythmics both in these patients and in heart failure of any other etiology due to their adverse effect on hemodynamics and prognosis. There is a high risk of sudden death in patients with malignant (life-threatening) ventricular arrhythmia (VVA), which includes sustained ventricular tachycardia, usually accompanied by severe hemodynamic disturbances, and ventricular fibrillation (after successful resuscitation). Most of these patients have significantly impaired left ventricular systolic function and low ejection fraction. The goal of treatment in patients with life-threatening ventricular arrhythmias is both to prevent recurrent VT and to prolong life expectancy. 80% of them are diagnosed with coronary artery disease and in a smaller number of cases, cardiomyopathy and valvular heart disease. In modern clinical practice, to determine the prognosis in patients with organic heart disease, in addition to sustained and unstable VT, ventricular extrasystoles (10 or more per hour), deterioration of myocardial contractility (EF less than 35-40%), indicators such as reduced variability are also taken into account cardiac rhythm, QT interval dispersion, T wave alternans. Left ventricular dysfunction and the possibility of inducing VT during electrophysiological studies are considered the most important predictors of poor prognosis.
Treatment of VT Relief of paroxysmal monomorphic VT Currently, it can be recognized that lidocaine is not the most effective drug for stopping VT, but it quickly begins to act and gives few complications. A number of studies have shown a higher effect of procainamide and sotalol. Comparing the effectiveness of intravenous administration of procainamide (10 mg/kg) and lidocaine (1.5 mg/kg) in relieving 41 episodes of sustained monomorphic VT A.R.M. Gorgels et al. [15] found that the effectiveness of novocainamide was significantly higher. It stopped 20 out of 26 episodes of VT, while lidocaine only 4 out of 15 (p In 1995, D. Scheinman et al. [16] published data from a randomized, double-blind study conducted in 46 US medical centers, which compared the effectiveness of various regimens IV administration of amiodarone in patients with life-threatening ventricular arrhythmias: IV drip infusion of amiodarone at a daily dose of about 1000 mg (after an initial rapid loading IV administration of 150 mg over 10 minutes) was 40-60% more likely to stop arrhythmias than with using the other two regimens studied. With intravenous administration of amiodarone, in addition, the effect of the drug appears quite late. In 50% of patients, the effect occurs within two to six hours from the start of drug use [17]. When stopping sustained VT, it is necessary to take into account how the effectiveness of the antiarrhythmic, as well as the state of myocardial contractile function. In this regard, to relieve monomorphic VT in patients with normal myocardial contractile function, it is proposed to use procainamide, sotalol, lidocaine and amiodarone, giving preference to the first two drugs, and in case of impaired myocardial contractile function (congestive cardiac failure or ejection fraction less than 40%) it is recommended to administer intravenously only lidocaine or amiodarone [17a]: • procainamide 1.0-1.5 g (up to 17 mg/kg): intravenous infusion at a rate of 30-50 mg/ min. To prevent relapses of VT, intravenous drip administration at a rate of 1-4 mg/min; • sotalol 1.0-1.5 mg/kg: intravenous administration at a rate of 10 mg/min; • lidocaine 1.0-1.5 mg/kg: intravenous administration over 2 minutes. If there is no effect and hemodynamics are stable, continue intravenous administration of 0.5-0.75 mg/kg every 5-10 minutes. In patients with congestive heart failure or EF less than 40%, it is advisable to use lower doses: 0.5-0.75 mg/kg IV over 2 minutes. If ineffective, repeat intravenous administration at the same dose every 5-10 minutes. The total dose of the drug administered in one hour should not exceed 3 mg/kg in all cases. In order to prevent recurrences of arrhythmia paroxysms, an intravenous infusion of lidocaine is performed at a rate of 1-4 mg/min; • amiodarone: 150 IV over 10 minutes, then infusion of 360 mg over 6 hours (1 mg/min) and 540 mg over the next 18 hours (0.5 mg/min). For recurrent arrhythmia, an additional 150 mg of amiodarone is administered intravenously over 10 minutes. The maximum total dose of an antiarrhythmic in 24 hours is 2.2 g. In patients with severe myocardial damage and a decrease in its contractile function, fractional intravenous administration of amiodarone in small doses is possible (150 mg every 10-15 minutes, the duration of each administration is 10 minutes) ; • if VT is not controlled by antiarrhythmic drugs or is complicated by severe hemodynamic disturbances, synchronized EIT is performed, in which the first shock power is 50-100 J.
Relief of paroxysmal polymorphic VT With a normal duration of the QT interval, relief of VT is carried out in almost the same way as in patients with monomorphic VT. When myocardial contractile function is preserved, lidocaine, amiodarone, procainamide, sotalol are used, and when myocardial contractility is reduced - lidocaine, amiodarone. If polymorphic VT develops in patients with acquired prolonged QT interval (tachycardia of the “pirouette” type, “torsades de pointes”), first of all it is necessary to discontinue drugs that could lead to prolongation of the QT interval (antiarrhythmics of classes 1 and 3, psychotropic drugs, antibiotics and etc.) and, if necessary, correct electrolytic disturbances (hypokalemia, hypomagnesemia). During attacks of arrhythmia that do not affect hemodynamics, ECG monitoring is carried out, and to reduce the risk of its relapse, the rhythm is increased to 90-110 per minute using a temporary pacemaker or IV infusion of isoproterenol. To relieve paroxysms of VT of the “pirouette” type, intravenous administration of magnesium sulfate (1-3 g over 2-5 minutes) or lidocaine (1-1.5 mg/kg over 2 minutes) is used, as well as EIT with the power of the first discharge not less than 200 J. According to the WHO drug monitoring center, the number of registered polymorphic VTs in patients with a long QT interval has increased significantly over the past 10 years. An analysis of 20 drugs most commonly associated with QT prolongation and torsades de pointes at this center showed that, as a percentage of the total number of adverse reactions, the antiarrhythmic drug ibutilide was the most common cause of this arrhythmia (24.9 %) and sotalol (4.7%), calcium antagonist bepridil (3.9%). For amiodarone and antiarrhythmic drugs of classes 1A and 1C, this ratio is significantly lower: for amiodarone – 0.34, quinidine – 0.45 and flecainide – 0.29% [18]. Thus, it can be stated that incidents of torsade de pointes tachycardia when using amiodarone are quite rare compared to other class 3 antiarrhythmic drugs. However, monitoring the duration of the QT interval when using antiarrhythmics of classes 1A, 1C and amiodarone is also necessary.
Prevention of relapses of sustained VT and the possibility of improving the prognosis in life-threatening (malignant) VT. For the preventive treatment of VT, antiarrhythmic drugs of classes 1 and 3 are mainly used. Class 2 antiarrhythmics (b-blockers) can be effective for VT that occurs during exercise, as well as in patients with congenital long QT syndrome. The class 4 antiarrhythmic verapamil prevents relapses of certain types of idiopathic VT. Rare, short-term, mild paroxysms of VT (usually idiopathic VT) do not require treatment. Evaluation of the effectiveness of pharmacotherapy for frequent symptomatic attacks of VT is carried out according to clinical data (the effect on the frequency of arrhythmia relapses), and for rare and severe paroxysms of VT - using special studies: non-invasive (Holter ECG monitoring in patients with frequent ventricular extrasystoles and/or runs of unstable VT , tests with dosed physical activity) and invasive (intracardiac EPS). With the help of long-term ECG monitoring, the possibility of suppression is determined, and with physical activity, the impossibility of inducing or increasing the frequency of ventricular arrhythmias against the background of oral administration of antiarrhythmic drugs. It has been established that in the case of positive results of non-invasive tests, prophylactic therapy with these drugs will prevent relapses of ventricular tachyarrhythmias (VT/VF) in most patients. Intracardiac EPS allows us to judge the effectiveness of an antiarrhythmic drug by its ability to provoke VA paroxysm by endocardial stimulation of the right ventricle. The importance of non-invasive and invasive testing in predicting the effectiveness of pharmacotherapy is approximately the same. However, according to some data, EPI is more sensitive in detecting the arrhythmogenic effects of antiarrhythmic drugs and therefore ensures greater safety of their use [18a]. From the table Table 2 shows that amiodarone and sotalol are more effective than class 1 antiarrhythmics in preventing recurrences of life-threatening VAs and increasing survival, and implantable cardioverter defibrillators, in turn, are superior to these class 3 antiarrhythmics in improving life prognosis. Therefore, for patients who have survived circulatory arrest (ventricular fibrillation) or have sustained VT with severe hemodynamic disturbances and poor contractile function of the left ventricular myocardium (EF) Pharmacotherapy of malignant ventricular arrhythmias (MVA) should begin with amiodarone or sotalol, and if there is no effect from them or the presence of contraindications, it is necessary to test other antiarrhythmics (including class 1) and, probably, combinations of antiarrhythmic drugs.It was previously indicated that amiodarone relatively rarely leads to the development of severe arrhythmogenic effects and, above all, VT (including type "pirouette"), despite the prolongation of the QT interval. Sotalol, being a highly effective antiarrhythmic [24], can cause a number of complications, mainly in the first three to four days from the start of treatment (symptomatic bradyarrhythmias - 10-15%, ventricular arrhythmias - 4- 5%) [25, 26]. Therefore, these days, careful ECG and clinical monitoring are necessary, especially when prescribing large doses of an antiarrhythmic, since the proarrhythmic effects of sotalol are dose-dependent. It is possible to select antiarrhythmic therapy using antiarrhythmic drugs of all classes only in 50-60% of patients with life-threatening ventricular arrhythmias [18a]. The effectiveness of pharmacotherapy decreases in proportion to the decrease in the contractile function of the left ventricular myocardium. In patients who have suffered cardiac arrest and who have been prescribed antiarrhythmic therapy (impossibility of re-inducing PVA using EPI), mortality during long-term follow-up (up to three years) ranges from 0 to 22% (on average 9%). When refractory to drug treatment – from 22 to 78% (average 43%) [27]. If pharmacotherapy for PAD does not produce positive results, it is necessary to evaluate the possibilities of non-drug treatment methods. Radiofrequency catheter ablation is most effective for certain types of idiopathic VT. According to the North American Society for Stimulation and Electrophysiological Research (NASPE, 1992), the effectiveness of RCD is 85%. In patients with post-infarction cardiosclerosis and dilated cardiomyopathy, the number of positive results is significantly less (about 50%), therefore, ICD is often used in them as an addition to implantation of a cardioverter-defibrillator to reduce the frequency of VT paroxysms and, consequently, the number of ICD discharges. Surgical treatment of VT is primarily indicated for patients with segmental myocardial damage (post-infarction scar, aneurysm) and preserved contractile function of the left ventricle. When there is congestive heart failure and/or low ejection fraction, which is the majority of patients with PVD, the results of surgical intervention (aneurysmectomy, endocardial ventriculotomy, subendocardial resection) are significantly worse. In this regard, surgical treatment of VAD in developed countries is currently used relatively rarely, and preference is given to implantation of cardioverter-defibrillators, performed without thoracotomy. The first report on implantation of a cardioverter-defibrillator was published by M. Mirowski et al. in 1980 [28]. By 1995, CDs had been implanted in more than 150 thousand patients. Surgical mortality for implantation without thoracotomy is low and ranges from 0 to 1.3% (average less than 0.5%). According to experts from the American College of Cardiology and the Heart Association [29], implantation of a cardioverter-defibrillator is the most effective method of treating PAD. The main indications for implantation of a cardioverter-defibrillator are: 1) cardiac arrest caused by VF or VT, but not associated with transient or reversible causes (level of evidence A); 2) spontaneous sustained VT in patients with organic heart disease (level of evidence B); 3) syncope of unknown origin, in which sustained VT with hemodynamic disturbances or VF is induced using EPS, and pharmacotherapy is ineffective or there is drug intolerance (level of evidence B); 4) unsustained VT in patients with coronary artery disease who have suffered an MI and have LV dysfunction, in whom VF is induced during electrophysiological studies or sustained VT that is not controlled by class 1 antiarrhythmics (level of evidence B); 5) patients with LVEF no more than 30% remaining one month after MI or three months after myocardial revascularization surgery. Implantation of a cardioverter-defibrillator is not recommended: 1) for patients in whom the trigger for arrhythmia can be identified and eliminated (electrolyte disturbances, overdose of catecholamines, etc.); 2) patients with Wolff-Parkinson-White syndrome and atrial fibrillation complicated by ventricular fibrillation (they should undergo catheter or surgical destruction of the accessory tract); 3) patients with ventricular tachyarrhythmias, which can be provoked by electrical cardioversion; 4) patients with syncope of unknown cause in whom ventricular tachyarrhythmias are not induced during electrophysiological studies; 5) with continuously recurrent VT or VF; 6) with VT or VF that can be treated with catheter ablation (idiopathic VT, fascicular VT).
References 1. Myerburg RJ, Kessler KM, Kimura S. Life-threatening ventricuar arrhythmias: the link between epidemiology and pathophysiology. In Zipes DP, Jalife J (eds). Cardiac electrophysiology. Philadelphia: WB Saunders company. 1994, p. 723. 2. Gomes JA, Winters SL, Stewart D. et al. A new noninvasive index to predict sustained ventricular tachycardia and sudden death in the first year after miocardial infarction: Based on signal-averaged electrocardiogram, radionuclide ejection fraction, and Holter monitoring. J. Am. Coll. Cardiol. 1987; 10: 349-357. 3. Bigger JT Identification of patients at high risk for sudden cardiac death. Am. J. Cardiol. 1984; 54: 3D-8D. 4. Cardiac arrhythmia suppression trial (CAST) investigators. Preliminary report: effect of encainide and flecainide on mortality in a randomized trial of arrhythmia suppression after myocardial infarction. N.Engl. J. Med. 1989; 321:406-412. 5. Effect of the antiarrhythmic agent moricizine on survival after myocardial infarction. The cardiac arrhythmia suppression trial II investigators. N.Engl. J. Med. 1992; 327: 227-233. 6. Morganzoth J., Goin JE Qinidine-related mortality in the short-to-medium term treatment of ventricular arrhythmias: a meta-analysis. Circulation. 1991; 84: 1977-1983. 7. Effect of prophylactic amiodarone on mortality after acute myocardial infarction and in cogestive heart failure: meta-analysis of individual data from 6500 patients in randomized trials. Lancet. 1997; 350: 1417-1424. 8. Doval HC, Nul DR, Grancelli HO et al. Nonsustained ventricular tachycardia in severe heart failure. Independent markers of increased mortality due to sudden death. GESICA-GEMA investigators. Circulation. 1996; 94: 3198-3203. 9. Singh SN, Fletcher RD, Fisher SG et al. Amiodarone in patients with congestive heart failure and asymptomatic ventricular arrhythmia. Survival Trial of Antiarrhythmic Therapy in Congestive Heart Failure. N.Engl. J. Med. 1995; 333: 77-82. 10. Boutitie F., Boissel JP., Stuart J. et al. Amiodarone interaction with b-blockers. Analysis of merged EMIAT and CAMIAT databases. Circulation. 1999; 99: 2268-2275. 11. A randomized trial of propranolol in patients with acute myocardial infarction. JAMA 1982; 247: 1707-1714. 12. Julian DG, Camm AJ, Frangin G. et al. Randomized trial of effect of amiodarone on mortality in patients with left-ventricular dysfunction after recent myocardial infarction: EMIAT. Lancet. 1997; 349:667-674. 13. Cairns JA, Connoly SJ, Roberts R., Gent M. Randomized trial of outcome after myocardial infarction in patients with frequent or repetitive ventricular premature depolarisations: CAMIAT. Lancet. 1997; 349: 675-682. 14. Moss AJ, Hall WJ, Cannom DS et al. Improved survival with an implanted defibrillator in patients with coronary disease at high risk for ventricular arrhythmia. N.Engl. J. Med. 1996; 335: 1933-1940. 14a. Julian DG, Prescott RJ, Jackson FS, Szekely D. Controlled trial of sotalol for one year after myocardial infarction. Lancet. 1982: 1142-1147. 14b. Sager PT New advances in class III antiarrhythmic drug therapy. Gurr. Opin. Cardiol. 2000; 15: 41-53. 14th century Moss AJ, Zareba W, Hall WJ et al. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduce injection fraction. N. Engl. J. Med. 2002; 346:877-883. 15. Gorgels APM, Adri van den Dool, Hofs A. et al. Comparison of procainamide and lidocaine in terminating sustained monomorphic ventricular tachycardia. Am. J. Cardiol. 1996; 78: 43-46. 16. Scheinman MM, Levine JH, Cannom DS et al. Dose-ranging study of intravenous amiodarone in patients with life-threatening ventricular tachyarrhythmias. Circulation. 1995; 92: 3264-3272. 17. Moos AN, Mohiuddin SM, Hee TT et al. Efficacy and tolerance of high-dose intravenous amiodarone for recurrent refractory ventricular tachycardia. Am. J. Cardiol. 1990; 65: 609-614. 17a. Guidelines 2000 for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. 2000; 102 (Suppl I): I-158-165. 18. Darp_ B. Spectrum of drugs prolonging QT interval and the incidence of torsades de pointes. Eur. Heart J. Supplements 2001; 3 (Suppl K): K70-K80. 18a. Golitsyn S.P. Treatment of ventricular arrhythmias from the perspective of primary and secondary prevention of sudden death // Heart failure. 2001. No. 2. P. 201-208. 19. Mason JW A comparison of seven antiarrhythmic drugs in patients with ventricular tachyarrhythmias. N.Engl. J. Med. 1993; 329:452-458. 20. Greene HL The CASCADE study: randomized antiarrhythmic drug therapy in survivors of cardiac arrest in Seattle. CASCADE investigators. Am. J. Cardiol. 1993; 72: 70F-74F. 21. Siebels J., Cappato R., Ruppel R. et al. Preliminary results of the cardiac arrest study Hamburg (CASH). CASH investigators. Am. J. Cardiol. 1993; 72: 109F-113F. 22. A comparison of antiarrhythmic-drug therapy with implantable defibrillators in patients resuscitated from near-fatal ventricular arrhythmias. The Antiarrhythmics Versus Implantable Defibrillators (AVID) investigators. N.Engl. J. Med. 1997; 337:1576-1583. 23. Cappato R. Secondary prevention of sudden death: the Dutch Study, the Antiarrhythmics Versus Implantable Defibrillators Trial, the Cardiac Arrest Study Hamburg, and the Canadian Implantable Defibrillator Study. Am. J. Cardiol. 1999; 83: 68D-73D. 24. Kehoe RF MacNeil DJ, Zheutlin TA et al. Safety and efficacy of oral sotalol for sustained ventricular tachyarrhythmias refractory to other antiarrhythmic agents. Am. J. Cardiol. 1993; 72:56A-66A. 25. Soyka LF, Wirtz C., Spangenberg RB Clinical safety profile of sotalol in patients with arrhythmias. Am. J. Cardiol. 1990; 65: 74A-81A. 26. Chung MK, Schweikert RA, Wilkoff BL et al. In hospital admission for initiation of antiarrhythmic therapy with sotalol for atrial arrhythmias required? JACC. 1998; 32: 169-176. 27. Myerburg RJ, Castellanos A. Clinical trials of implantable defibrillators. N.Engl. J. Med. 1997; 337: 1621-1623. 28. Mirowski M, Reid PR, Mower MM et al. Termination of malignant ventricular arrhythmias with an implanted automatic defibrillator in human beings. N.Engl. J. Med. 1980; 303: 322. 29. ACC/AHA guidelines for implantation of cardiac pacemakers and antiarrhythmia devices. Circulation. 2002; 106: 2145-216.
Atrial fibrillation
The main ECG sign of atrial fibrillation is significantly different adjacent RR intervals in the absence of an atrial P wave. With a resting ECG, minor fluctuations in the isoline are very likely to be recorded (atrial fibrillation itself), however, with Holter recording, interference can level out this sign.
↓Start an episode of atrial fibrillation after normal sinus rhythm (from the fifth complex). Tachysystolic form.
↓Atrial fibrillation itself is visible (jagged isoline) - according to old classifications, “large-wave” - in the chest leads. Bradysystole. Complete block of the right bundle branch ("ears" in V1-V2)
↓ “Small wave”, according to old classifications, atrial fibrillation, visible in almost all leads.
↓Rhythmogram for persistent atrial fibrillation: there are no two equal adjacent RR intervals.
↓Rhythmogram when fibrillation changes to sinus rhythm with a lower heart rate in the middle of the picture - an episode of sinus rhythm. At the beginning of an episode of sinus rhythm, the sinus node “thinks” whether to turn on or not, hence the long pause.
↓The heart rate trend in atrial fibrillation is very broad, often with a high average heart rate. In this case, the patient has an artificial pacemaker programmed for 60 beats per minute, so all frequencies below 60 beats per minute are “cut off” by the pacemaker.
↓Heart rate trend in paroxysmal atrial fibrillation. Signs of AF are a “high” and “broad” trend, sinus rhythm is a narrow band located significantly “lower”.
Folk remedies
Photo: rusteaco.ru
For ventricular tachycardia, the use of traditional methods of treatment is possible as an addition to drug therapy prescribed by a doctor. Tinctures and herbal infusions will help reduce the heart rate, but do not forget that they act very gently, so they are used outside of exacerbations of the disease. The most popular plants are:
- Melissa. The tincture is taken 4 teaspoons per day, after diluting them with 50 ml of water.
- Elecampane. The roots of the plant are used to prepare the tincture. Dosage: 1 teaspoon 3 times a day 15 minutes before meals.
- Valerian. Chopped roots (1 tbsp) are poured with water and infused. Take 100 ml once a day.
The information is for reference only and is not a guide to action. Do not self-medicate. At the first symptoms of the disease, consult a doctor.
Treatment of tachycardia
In the case when tachycardia occurs once and quickly stops on its own, this should not be a cause for concern. But if this symptom appears constantly, then you need to consult a cardiologist. It should be remembered that tachycardia can be a symptom of both a functional disorder of the nervous system and a serious heart disease.
Depending on the indications, cardiologists at the CELT clinic conduct examinations to identify the cause of the patient’s tachycardia:
- ECG;
- Holter ECG monitoring;
- Stress test;
- Transesophageal ECG and atrial stimulation;
- EchoCG;
- Study of sugar metabolism disorders;
- Tests for the activity of thyroid hormones.
Having determined the cause of the increased heart rate, the doctor will be able to choose the optimal treatment regimen for the underlying disease.
Make an appointment through the application or by calling +7 +7 We work every day:
- Monday—Friday: 8.00—20.00
- Saturday: 8.00–18.00
- Sunday is a day off
The nearest metro and MCC stations to the clinic:
- Highway of Enthusiasts or Perovo
- Partisan
- Enthusiast Highway
Driving directions