Portal vein thrombosis (PVT), or pilethrombosis, is a rarely diagnosed vascular disease of the liver and can be the result of a large number of different diseases, both surgical and therapeutic, remaining asymptomatic for a long period of time, which makes its timely diagnosis difficult. At the same time, the prognosis for PVT is always serious and unfavorable; in half of the cases, deaths are observed due to gastrointestinal bleeding and progression of portal hypertension (PH) [6, 9]. PVT is the process of thrombus formation up to complete occlusion of the lumen of the main trunk and branches of the PVT with progressive impaired blood flow in the liver and gastrointestinal tract.
For the first time, an intravital diagnosis of thrombosis of the main trunk of the venous vein was made by S.P. Botkin. in 1862 [1, 7]. The first description of PVT belongs to Balfur and Stewart (1868) using the example of a patient with splenomegaly, ascites and varicose veins (VV) [1, 3]. In 1934, Strazhesko N.D. Based on his own research and literature data, he developed and described the main symptoms of intravitally recognized PVT [3].
There is no reliable information about the frequency of PVT. Depending on diagnostic methods and patient sampling criteria, statistical data vary greatly. According to autopsy data, in the USA the incidence of portal thrombosis ranges from 0.05 to 0.50% [9, 15]; Data from Japanese pathologists confirm these figures. In the European population, PVT accounts for up to 10% of all cases of PG, while in developing countries this figure reaches 40% [5, 12]. The incidence of PVT in patients with liver cirrhosis (LC), according to various literature data, ranges from 1 to 43% [5, 9]. During liver transplantation, the incidence of PVT varies from 2 to 26% [5].
Thus, the problem of PVT is of great medical importance, especially since there are still no carefully developed algorithms for diagnosing and treating this pathology.
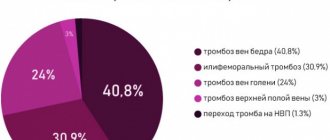
BB system
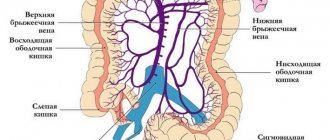
The PV system includes all the veins that carry out the outflow of venous blood from the intra-abdominal part of the gastrointestinal tract, spleen, pancreas and gall bladder (see figure).
. Anatomical structure of the portal vein system. S. Sherlock, J. Dooley.
The PV is formed from the confluence of the superior mesenteric and splenic veins behind the head of the pancreas at approximately the level of the second lumbar vertebra; the length of the PV at the hepatic portal is 5.5–8.0 cm. At the porta hepatis, the PV is divided into the right and left lobar branches, corresponding to the lobes of the liver; further in the liver, the AV is divided into segmental branches accompanying the branches of the hepatic artery.
The BB does not contain valves in the main branches. The distribution of portal blood flow in the liver is not constant: blood flow to the left or right lobe of the liver may predominate. In humans, it is possible for blood to flow from the system of one lobar branch to the system of another. The pressure in a person's ventricular vein is normally about 7 mm Hg. Art. The volumetric velocity of blood flow through IVs reaches 1000–1200 ml/min [3].
Etiology and pathogenesis of PVT
According to modern concepts, venous thrombosis is the cumulative result of congenital or acquired procoagulant disorders and the action of local factors [10, 22].
A condition in which the balance between the processes of coagulation and fibrinolysis is disturbed in favor of coagulation processes can be defined as thrombophilia. A person with congenital or acquired thrombophilic disorders during life more often than in the normal population develops arterial or venous thrombosis of various locations (Table 1) [5, 11,12, 14, 23].
The Leiden mutation in the factor V gene occurs in 2–4% of the European population and leads to a change in the amino acid sequence of coagulation factor V itself (replacement of arginine with glutamine at position 506). Mutated factor V is resistant to destruction by protein C; constant circulation of activated factor V leads to uncontrolled synthesis of thrombin and the formation of blood clots [11, 13, 16, 23].
The mutation of the coagulation factor II gene was first described in 1996, occurs in the general population in 2% of the population and leads to excessive generation of prothrombin, i.e., a shift in hemostasis to the procoagulant side [17, 22].
Protein C is synthesized in the liver through a vitamin K-dependent mechanism and is a natural anticoagulant. Protein S is a cofactor of protein C, involved in the neutralization of active forms of coagulation factors V and VIII. Currently, about 160 mutations of the protein C gene and many mutations of the protein S gene have been described, which leads to a deficiency of these proteins and the development of thrombophilia [11].
Acquired blood coagulation disorders can occur in myeloproliferative diseases, antiphospholipid syndrome; they also accompany inflammatory and oncological diseases and are observed during the use of oral contraceptives [16, 22, 23], pregnancy and hyperhomocysteinemia. In 60% of patients with thrombosis of the veins of internal organs, hypercoagulation is observed, in 25% the influence of local factors is of paramount importance [16, 20] . PVT is characterized by a combination of causative factors [18, 20]. In approximately 20% of cases, the development of thrombosis does not find an exhaustive explanation [21]. The occurrence of thrombosis is promoted by all diseases that occur with a slowdown in blood flow through the liver.
The pathogenesis of PVT in cirrhosis is not fully understood. PH, a decrease in the rate of blood flow through IVs, and peripheral lymphangiitis and fibrosis may be important here [20].
. Causes of thrombophilia.
Causes of thrombosis
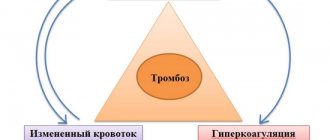
In order for a blood clot to form in a vein, a number of conditions or factors are necessary. In the classical version, there are three factors that contribute to thrombus formation (Virchow’s triad).
- hypercoagulable state (increased coagulability). There are many reasons for increased blood clotting - surgery on any part of the body, pregnancy, childbirth, diabetes, excess dietary fat, oral contraceptives, dehydration, genetic factors (rare), etc.
- injury to the inner wall of the vessel (endothelium). The vascular wall can be injured during the installation of a venous catheter, injection into a vein (due to this, thrombosis is very common in drug addicts who inject into the veins of the legs, in the groin), injuries, during radiation therapy, chemotherapy, etc.
- slowing down blood flow. It can be observed in various conditions - varicose veins, pregnancy, obesity, immobilization of a limb (for example, when wearing a cast after fractures), heart failure, forced sedentary position of the body (for example, during long air travel), compression of veins by tumors, etc.
Thrombosis can occur due to the action of one factor or a combination of them. For example, in case of a fracture of the leg bones, all three factors can be involved - increased coagulability in the case of extensive hemorrhages in the area of injury, damage to the vascular wall as a result of a mechanical shock, and a slowdown in blood flow as a result of wearing a cast.
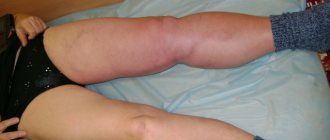
Most often, thrombosis occurs in the veins of the lower extremities. This is due to the fact that stagnation most often occurs in these veins (obesity, varicose veins, edema, etc.).
TVV Clinic
The clinical picture depends on the location and extent of PVT, the speed of its development and the nature of the predisposing liver disease. PVT can be divided into acute and chronic based on the rate of development of venous obstruction. Thrombosis of the PV or its branches should be considered in all cases of sudden onset of pain in the epigastric region, combined with severe bloating, rapid accumulation of fluid in the peritoneal cavity, and repeated vomiting. PVT can manifest itself at any age: from 6 weeks to old age; There are no differences in the frequency of its occurrence between children and adults [5]. Clinical manifestations can range from a completely asymptomatic course of the disease to an acute onset with hematemesis. Bleeding from varicose veins of the esophagus and rectum is the most common manifestation of PVT. In the presence of splenomegaly and hypersplenism, thrombocytopenia, abdominal pain, transient or permanent ascites, lack of appetite, weakness, and weight loss are observed.
Chronic PVT can manifest itself only with nonspecific symptoms - general weakness, lack of appetite, and in the absence of proper diagnosis, which is quite common, it can be missed.
In therapeutic practice, it is more common to observe sluggish forms of pylephlebitis against the background of portal thrombosis. They are characterized by mild pain in the liver area, moderate leukocytosis, and prolonged low-grade body temperature. The slightly enlarged liver is sensitive to palpation. The blood in cases of sluggish pylephlebitis turned out to be sterile. The disease is usually mistaken for chronic cholecystocholangitis, chronic appendicitis or other chronic inflammatory disease of an internal organ. The cause of fever usually becomes obvious only after long-term observation, during which the patient develops an enlarged spleen and signs of more or less developed collateral circulation.
The following stages of PVT are distinguished: 1. Acute: thrombus formation; BB can be increased. 2. Subacute: a thrombus and small venous collaterals are visualized; the diameter of the explosive can be increased. 3. Chronic: cavernous transformation of the PV; development of large collaterals in the projection of the obliterated PV; The PV is reduced in diameter or is not visualized.
Depending on the location of the thrombus, three types of PVT are distinguished: • radicular form (thrombosis of the splenic vein and mesenteric vessels); • terminal form (thrombosis of small branches and capillaries of IVs in the liver); • trunk thrombosis (in the PV trunk itself).
The blood supply to the intestine above the level of the PVT (along the blood flow) remains satisfactory as long as circulation through the arcuate vessels of the intestine is maintained. If they thrombose, intestinal ischemia develops. This is probably facilitated by the absence of vascular collaterals. The consequence of ischemia can be intestinal necrosis with the development of peritonitis, multiple organ failure, and in a quarter of cases, death even after resection of the necrotic section of the intestine [9, 21].
On the contrary, below the level of thrombosis, clinically significant liver dysfunction does not develop, since its blood supply is maintained due to an increase in blood flow through the hepatic artery (due to the expansion of its branches) and the development of portal collaterals, from which a cavernoma is formed [7, 19]. Collateral veins develop over several days and form a cavernoma, which during diagnosis is often misinterpreted as a vascular tumor or developmental anomaly. Collateral veins can change the appearance of anatomical structures adjacent to the thrombosed area of the venous vein, in particular the bile ducts, gallbladder, pancreas, antrum of the stomach, and duodenum. During diagnostic studies, this leads to erroneous diagnosis of tumors, pancreatitis, and cholecystitis. In addition, changes in the bile ducts caused by the development of collaterals can lead to the development of jaundice. The pressure in the explosive system increases; the picture of PG develops.
Ultrasonography
Portal vein thrombosis (PVT) is increasingly recognized by ultrasound. Abdominal sepsis and decreased portal blood flow resulting from parenchymal liver disease are the main causes.
Figure 8 : Portal vein thrombosis. Longitudinal oblique sonogram of a 36-year-old woman with a history of Leber optic atrophy (hereditary optic neuroretinopathy) and alcohol abuse who had nonspecific complaints of malaise and vague abdominal pain. The image shows ascites and a bright liver (obesity). The portal vein has a linear echogenic structure running along the length of the portal vein (solid arrow). There is a cystic formation in the liver (open arrow).
Figure 9 : Portal vein thrombosis. Power Doppler of the liver shows blood flow around an intraluminal filling defect in the portal vein (P).
Figure 10 : Portal vein thrombosis. A spectral Doppler sonogram of the liver obtained from the same patient as in the previous image shows the portal vein (cursor) which does not demonstrate blood flow.
Figure 11 : Portal vein thrombosis. A longitudinal oblique sonogram was obtained from a 28-year-old woman who was referred for a gallbladder ultrasound. The image shows several vascular tubular structures at the porta hepatis, which are suggestive of cavernous transformation.
Figure 12 : Portal vein thrombosis. A color Doppler sonogram obtained from the same patient as in the previous image shows blood flow in the cavernous mass.
Figure 13 : Portal vein thrombosis. Color Doppler ultrasound of the spleen obtained from the same patient as in the previous 2 images shows mild splenomegaly with splenic varices. Endoscopic findings confirmed the presence of esophageal varices.
Figure 14 : Color Doppler ultrasound depicts a vascularized portal thrombus.
Figure 15 : Color Doppler ultrasound depicts a vascularized portal thrombus.
Figure 16 : Echoic partially recanalized portal vein thrombus. The patient is a 36-year-old woman with idiopathic chronic portal vein thrombosis of 2 years duration. She has cholestatic jaundice.
Sonograms may show echogenic lesions in the portal vein. A clot with variable echogenicity may be depicted. The clot is usually moderately echogenic, but if it has recently formed, it may be hypoechoic.
Patent vessels may have increased intraluminal echogenicity due to the accumulation of red blood cells, making slow blood slightly echogenic. Increased or decreased echogenicity may be observed in the lumen of the portal vein.
PVT eliminates the normal venous flow signal from the portal vein lumen during pulsed or color Doppler ultrasound. Color Doppler flow images can show flow around a clot that is partially blocking a vein. However, if the flow is slow, the Doppler signal may not be detected. Color flow may be present in other small collateral vessels.
Incomplete occlusion may occur. This is typical for tumor lesions. Alternatively, thrombolytic recanalization may occur. Cavernous malformations, spontaneous shunts, splenorenal and portosystemic collaterals can also be detected. The underlying cause may be obvious: hepatocellular carcinoma, metastases, cirrhosis, pancreatic neoplasms.
It is assumed that the thickening of the portal vein with a narrowing of its lumen is caused by portal phlebitis. This is considered a precursor to PVT in patients with acute pancreatitis. The diameter of the portal vein is greater than 15 mm in 38% of cases of PVT.
Diagnosis of PVT
The need to use imaging studies is determined by the lack of specific signs of PVT and the need to identify the level of venous outflow impairment. The main diagnostic methods in this case are ultrasound (US) and Doppler studies, computed tomography (CT), magnetic resonance imaging (MRI) and angiography.
Ultrasound can detect PVT only when areas of increased echogenicity caused by the presence of blood clots are visualized in the lumen of the PVT. With an increase in the size of the PV, one can only assume PG, i.e. this sign is not diagnostic. Detection of venous collaterals more reliably confirms the diagnosis of PG.
Doppler ultrasound makes it possible to identify the structure of the PV and hepatic artery. The diagnostic value and results of the study depend on the experience of the physician and the quality of the analysis of image details. The greatest difficulty is in studies of cirrhotic livers and in overweight patients [2, 19]. With color Doppler mapping (CDC), the quality of PVT visualization improves. Technically correctly performed colorectal dizziness allows diagnosing ventricular obstruction as reliably as angiography. With CDK it is possible to identify natural intrahepatic portosystemic shunts. This is the cheapest, most effective and non-invasive method. Its high sensitivity and specificity are observed in patients with extensive thrombotic lesions and complete closure of the vascular lumen. At the same time, the presence of residual blood flow along the IV increases the likelihood of a technical error when assessing the results of the study [5, 19].
In order to reduce the risk of such errors when diagnosing PVT, duplex ultrasound scanning is used to determine the velocity of portal blood flow. This method combines Doppler ultrasound scanning with traditional ultrasound, which allows the doctor to see the structure of the blood vessels. Duplex scanning shows the movement of blood through the vessels and allows you to measure the speed of blood flow. This method also allows you to determine the diameter of blood vessels and identify their blockage. The most modern and accurate imaging methods include CT, venography and MRI.
CT can provide information about the condition of the vessel walls, liver damage (for example, with the formation of cavernous nodes) and show the extent of thrombosis [5, 15].
After administration of a contrast agent, it becomes possible to determine the lumen of the venous veins and identify ventricular veins located in the retroperitoneal space, as well as perivisceral and paraesophageal veins. The esophageal esophagus bulges into its lumen, and this bulge becomes more noticeable after the administration of a contrast agent.
CT in combination with arterial portography makes it possible to identify collateral blood flow pathways and arteriovenous shunts. Portography through the internal jugular vein is an invasive but very accurate diagnostic method. It allows you to assess the extent of thrombosis, its exact location and the severity of thrombotic stenosis of the PV.
MRI allows you to very clearly visualize and study blood vessels. This method is used to determine the lumen of shunts, as well as to assess the consistency of portal blood flow. MRI results are more reliable than Doppler ultrasound data [5, 15, 19].
Venography is used to verify the diagnosis in cases where the above-described research methods do not clearly confirm or exclude the suspected PVT. At the same time, if the patency of the vein is established by any method, confirmation of this using venography is not mandatory. Venography also makes it possible to detect defects in the filling of the PV or its branches, indicating external compression by a space-occupying formation [5].
In clinical practice, for the diagnosis of portal thrombosis, two methods are usually combined to overcome the disadvantages of each of them. When diagnosing PVT, you should pay attention to the coagulogram: an increase in fibrinogen content, the appearance of activated fibrinogen, an increase in the prothrombin index, a decrease in blood clotting time.
Preferred research methods
Preferred studies include duplex Doppler and/or color Doppler, computed tomography, magnetic resonance angiography, and arterial portography or splenoportography.
Figure 1 : Portal vein thrombosis. Superior mesenteric angiogram shows collateral vessels at the portal portion of the liver, but no patent portal vein. Note that ascites causes the liver to move away from the chest. This patient had severe liver failure and died 72 hours after imaging. Post-mortem examination showed early cirrhosis, fulminant pyogenic cholangitis, multiple liver abscesses, thrombosis of the portal vein and spleen and left gastric vein.
A tumor in the portal vein may have an appearance identical to thrombosis, but this type is much less common than others. The thrombus may be partial or complete. It can also be mixed with soft clot.
Adults in whom acute PVT is secondary to abdominal sepsis may recover fully and the vessel can be reanalyzed with successful treatment of the underlying sepsis.
Failure to visualize the portal vein strongly suggests occlusion. In this case, the portal vein can be viewed as a band of high-level echoes at the portal portion of the liver.
The development of PVT may precipitate the need for emergency endoscopy for variceal sclerotherapy, TIPS, surgical creation of a portacaval shunt, transjugular or transhepatic portomesenteric thrombolysis, and thrombectomy or even resection. However, PVT may complicate sclerotherapy. Fine needle aspiration biopsy of PVT can be performed under color Doppler sonographic guidance to evaluate therapeutic efficacy.
Early complications of transjugular intrahepatic portosystemic shunt (TIPS) that can be detected by ultrasonography include the following:
- intraperitoneal hemorrhage,
- shunt thrombosis,
- neck hematoma,
- disturbance of blood supply to the liver,
- TVV,
- hepatic artery occlusion,
- liver infarction,
- failed stent deployment
- inadequate expansion of the stent,
- stent expansion,
- obstruction of the bile ducts.
Investigators reviewing the results of patients with cirrhosis undergoing TIPS noted that although an increase in portal blood flow velocity and a decrease in portal hypertension are achieved, the hypercoagulable state persists and may cause expansion of the residual PVT or rethrombosis.
PVT can also interrupt liver perfusion, causing hepatocyte ischemia and hormonal deprivation, which can lead to hepatocyte death, loss of parenchyma, and ultimately worsening liver fibrosis and function, leading to increased mortality.
Treatment of PVT
Management of patients with PVT depends on the onset of the disease (acute or chronic), clinical presentation, etiological factors leading to PVT, age and other factors. Complications of PVT - ascites, bleeding from PVT - are treated in the same way as for cirrhosis with PG.
In acute PVT, anticoagulant therapy is recommended, which leads to complete or partial restoration of the PVT lumen in 70–80% of patients [5, 12, 15].
Spontaneous thrombus lysis in PVT is possible, but is very rare. Anticoagulant therapy does not increase the risk of bleeding, but it reduces the risk of intestinal infarction, which is life-threatening. The recurrence rate of PVT varies from 6 to 40% [2, 21], so some authors recommend anticoagulant therapy for 6 months [10, 21].
Thrombolysis through a transhepatic approach for acute PVT is a method that avoids the side effects of systemic anticoagulant therapy. Thrombolysis can also be carried out systemically, especially in cases of acute total or subtotal occlusion of IVs [9, 10].
Unfortunately, there is still no clear practical guidance on the prescription of anticoagulants in patients with PVT. How to choose the right anticoagulant? What doses should be used? When to stop anticoagulant therapy? What contraindications exist for the use of anticoagulants? Large randomized trials are needed to answer these questions.
Surgical treatments include transhepatic angioplasty, thrombolysis followed by transjugular intrahepatic portosystemic shunt (TIPS), thrombectomy with local fibrinolytic infusion. If the patient is planning to undergo shunting, it is preferable to perform distal splenorenal shunting [5, 10]. Splenectomy should be avoided, since it itself is an etiological factor in the development of PVT. The main methods of medical and surgical treatment of PVT are presented in Table. 2.
. Basic methods of treating PVT.
Portal vein thrombosis: a modern view on the issues of etiopathogenesis, prevention and treatment
Portal vein thrombosis (PVT) is a form of venous thrombosis that causes disruption or cessation of blood flow in the portal vein. The prevalence of PVT is approximately 1% in the general population and is most common in the setting of liver cirrhosis, inflammatory abdominal disease, and hepatocellular carcinoma. There are complete and partial thrombosis, as well as acute and chronic forms of PVT. The acute form can be manifested by ischemia and infarction of the intestine due to the spread of a blood clot to the mesenteric vessels. The chronic form is often asymptomatic due to the inclusion of compensatory mechanisms, such as dilatation of the hepatic artery and the development of a network of collaterals. Manifestations of chronic PVT may include bleeding from the gastrointestinal tract as a consequence of portal hypertension and biliopathy. Diagnostic methods for PVT include Doppler sonography, computed tomography and magnetic resonance imaging with contrast. Anticoagulant therapy is considered the treatment of choice for acute PVT. In chronic PVT, monitoring and prevention of bleeding from esophageal varices (EVV) and, if necessary, drug thrombolysis are recommended. PVT is a predictor of mortality in liver transplantation.
Table. Systemic factors for portal vein thrombosis (AASLD, 2009)
Drawing. The main vessels of the portal system of the liver
Epidemiology
Portal vein thrombosis (PVT) involves complete or partial occlusion of the lumen of the vessel draining the gastrointestinal tract. PVT was first described in 1868 in a patient with splenomegaly, ascites, and esophageal varices (ESVV) [1].
PVT is diagnosed in 5–10% of patients with portal hypertension in developed countries and in 30–35% of patients in developing countries due to the high incidence of infectious complications predisposing to PVT [2].
The prevalence of PVT among patients with liver cirrhosis (LC), detected at autopsy, is 6–64%, according to the results of ultrasound diagnostics – 5–24% [3]. The risk of thrombosis is closely related to the severity of liver disease and is less than 1% of cases in patients with compensated cirrhosis, 8–25% of cases in patients who are candidates for liver transplantation [4, 5].
Based on 24,000 autopsies performed in Sweden between 1970 and 1982, the prevalence of PVT in the general population was 1%. At the same time, the CPU share accounted for 28%. The most common cause of thrombosis was malignant liver disease (primary – 23%, secondary – 44%). In 10% of cases, PVT was caused by an infectious and inflammatory disease of the abdominal organs, and in 3% by a myeloproliferative disease. Interestingly, in 14% of cases the cause of thrombosis was not identified [6].
Etiology
The etiological factors for the occurrence of a thrombus in the portal vein system are intra-abdominal (local), which account for about 70% of cases of PVT, and systemic - 30% of cases of PVT.
According to the American Association for the Study of Liver Diseases (AASLD) 2009, intra -abdominal
factors include [7]:
- cirrhosis of the liver;
- abdominal cancer;
- neonatal omphalitis. Umbilical vein catheterization;
- diverticulitis, appendicitis;
- pancreatitis;
- duodenal ulcer;
- cholecystitis;
- tuberculous lymphadenitis;
- inflammatory bowel diseases;
- cytomegalovirus hepatitis;
- mechanical trauma of vessels in the portal vein system;
- splenectomy, colectomy, gastrectomy, cholecystectomy;
- liver transplantation;
- abdominal trauma;
- transjugular intrahepatic portosystemic shunt, TIPS (transjugular intrahepatic portosystemic shunt).
For a hepatologist, the most important questions are: what explains the development of PVT in cirrhosis? Are there predictors of its development depending on the severity according to the Child–Pugh or MELD criteria? Speaking about the pathomorphological reasons for the development of PVT, it is necessary to remember that one of the main reasons is a decrease in blood flow in the portal vein due to progressive fibrosis. Recently, more and more data speak in favor of a “prothrombotic imbalance” that develops with cirrhosis (as opposed to traditional ideas about hepatic coagulopathy). Indeed, a violation of the protein-synthetic function of the liver is manifested by a decrease in the production of both anticoagulant and procoagulant factors (with the exception of factor VIII). The development of PVT is also facilitated by a decrease in the activity of thrombomodulin (a powerful anticoagulant, an activator of protein C), an increase in the level of procoagulant factor VIII in plasma, and ineffective fibrinolysis due to a decrease in the level of plasminogen and an increase in the level of plasminogen activator inhibitor [8, 9].
Malignant diseases of the abdominal organs (usually liver and pancreatic cancer) cause PVT in 21–24% of cases. This is due, on the one hand, to hypercoagulation associated with the neoplastic process, and on the other hand, to direct invasion or compression of the vessel by the mass of the tumor and impaired blood flow [7].
Less common intra-abdominal (local) factors include lymphadenopathy, systemic inflammatory response syndrome, vascular injuries during surgical interventions (TIPS, splenectomy, liver transplantation, ablation for hepatocellular carcinoma, etc.) [10].
To system
Factors in the development of PVT include a number of congenital and acquired conditions (see table).
Factor V and protein C mutations are the most common congenital pathologies predisposing to PVT. The role of protein S and antithrombin III deficiency is not fully understood. In patients with cirrhosis, a mutation of the prothrombin gene plays a certain role in the development of thrombosis (while in the general population it is considered clinically insignificant). Note that among systemic factors in the etiology of PVT, acquired thrombophilic disorders prevail compared to congenital genetic defects of coagulation [11]. There is a formula for determining the origin of coagulopathy in PVT in the presence of concomitant liver disease. If the ratio of protein C or protein S or antithrombin to (factor II + factor X)/2 is less than 70%, a genetically determined congenital anticoagulant deficiency is suspected to be the cause of PVT [10].
According to a number of authors, from 22 to 48% of cases of PVT are considered the primary manifestation of a myeloproliferative disease. The 1849G → T point mutation in the gene encoding the tyrosine-protein kinase JAK2 is highly specific and suggests the presence of myeloproliferative disease as the cause of PVT [7].
Pathophysiology of PVT and complications
As is known, the portal vein is formed by the confluence of the splenic and superior mesenteric veins. A thrombus can form in the intrahepatic part of the portal vein, which occurs in liver cancer and cirrhosis, with subsequent spread of the process to the extrahepatic part of the vessel.
Thrombosis can occur in the splenic vein against the background of an inflammatory process in the abdominal cavity (usually chronic pancreatitis) and spread to the portal vein. But usually the site of thrombosis is the portal vein at the junction of two veins (see figure) [12].
Complications of PVT are associated with the spread of a thrombus to the mesenteric veins and intestinal arcades. Thrombosis in the intestinal arcades, on the one hand, disrupts their functioning as a compensation mechanism as a result of collateral circulation that maintains normal blood supply to the small intestine; on the other hand, it can lead to a reflex narrowing of the arterioles with subsequent ischemia of the corresponding area of the small intestine. Severe complications of intestinal ischemia include intestinal infarction, which is observed in 5% of patients with acute PVT [13].
An interesting fact is that PVT does not lead to changes in liver function. In this case, as a rule, there are no deviations in the indicators indicating hepatosuppression. However, according to experimental studies, PVT can cause certain histological changes in the liver. Thus, ligation of the portal veins in rats led to apoptosis of hepatocytes and was accompanied by increased mitotic activity in the perfused areas. In this case, the severity of apoptosis depended on the degree of ligation of the portal vein. This phenomenon explains the positive effect of embolization of the branches of the portal vein during extensive liver resections: due to atrophy of the “embolized” area, hypertrophy of the remaining part of the liver occurs, as a result of which the effectiveness of surgical intervention increases [14, 15].
At the same time, mechanisms are described due to which, when portal blood flow is disrupted (it accounts for 2/3 of the blood supply to the liver), significant changes in liver function do not occur. First, in response to a decrease in portal blood flow, dilation of the hepatic artery is observed, which increases blood flow to the liver through the arterial bed. Secondly, a network of collaterals develops through which blood enters the liver, bypassing the “block” in the portal vein. Collateral veins take on the appearance of a cavernous vascular formation - a “portal cavernoma”, the formation period of which is on average five weeks. With endoscopic retrograde cholangiohepatography, portal vein cavernous malformation appears as a sponge-like mass under the liver. We should not forget that a cavernoma can cause compression of the bile ducts (portal biliopathy). Both intra- and extrahepatic bile ducts may be involved in the process. In most cases, portal biliopathy has no clinical manifestations, although in 10–20% of cases, especially in elderly patients, it can manifest as icteric syndrome, cholangitis, and cholecystitis [16, 17].
Clinical picture, diagnosis
Symptoms that can be observed in acute PVT
, varied: sudden acute pain in the abdomen, bloating, diarrhea, rectal bleeding, nausea, vomiting. Characteristically, there are signs of a systemic inflammatory response: febrile fever, increased levels of acute phase proteins. However, liver function tests remain within normal limits. If thrombosis does not spread to the mesenteric vessels, then the process is usually reversible due to the inclusion of compensatory mechanisms (collateral circulation) or the portal vein [7].
The clinician should remember that against the background of acute thrombosis, pylephlebitis may develop - purulent thrombophlebitis of the portal vein as a result of infection of organs drained by the portal vein or adjacent to it (for example, bile ducts). Pylephlebitis begins with thrombophlebitis of the veins draining foci of infection, spreading to the portal and mesenteric veins. In addition, the development of pylephlebitis is facilitated by occlusion of the vessel by thrombotic masses [18, 19].
In the clinical picture of chronic PVT
a number of syndromes and conditions can be distinguished: on the one hand, manifestations of portal hypertension predominate, while the first manifestation of chronic PVT in 20–40% of cases is considered to be bleeding from the urinary tract and the stomach, on the other hand, biliary colic, jaundice, cholangitis, cholecystitis, pancreatitis can develop (portal biliopathy), as well as hypersplenism with subsequent pancytopenia and encephalopathy, which can be considered as complications [7].
Chronic PVT is often asymptomatic. Its diagnosis is possible only in the event of complications or ultrasound examination of the abdominal cavity.
In the diagnosis of PVT, ultrasound plays an important role, the sensitivity and specificity of which are 60–100%. In this case, there is visualization of solid hyperechoic material in the lumen of the portal vein or its branches, the presence of collateral vessels and cavernoma. Doppler sonography reveals the absence of blood flow in the lumen of the vessel [18].
The sensitivity of endoscopic ultrasound is 81%, specificity is 93%. This study can detect small non-occlusive thrombi, as well as tumor invasion of the vessel. But this method has a “blind spot”: the distal part of the superior mesenteric vein and the intrahepatic part of the portal vein are not visualized [19].
Modern methods such as computed tomography (CT) and magnetic resonance imaging (MRI) make it possible not only to detect the presence of a blood clot, but also to identify the causes of thrombosis and diagnose complications (for example, ischemia and infarction of the intestine).
The AASLD guidelines recognize CT as the standard for diagnosing acute PVT. Thus, if acute PVT is suspected, it is recommended to perform a CT scan with contrast. In case of fever against the background of acute thrombosis, it is advisable to carry out bacteriological blood culture to detect septic pylephlebitis. If chronic PVT is suspected, Doppler sonography, CT, or MRI with contrast are recommended [20].
Treatment of PVT
The main directions of treatment when making a diagnosis:
- preventing the spread of thrombus through the mesenteric veins;
- achieving vessel recanalization;
- treatment of complications associated with portal hypertension (in particular, bleeding from varicose veins) and portal biliopathy.
In acute PVT
Drug thrombolysis is considered the treatment of choice. According to four retrospective studies, in patients with acute PVT who received anticoagulant therapy for six months, complete recanalization was achieved in 50% of cases, partial recanalization in 40%. Only 10% of patients were resistant to the therapy.
Anticoagulant therapy is indicated primarily for concomitant mesenteric vein thrombosis. According to some experts, in case of intestinal infarction, anticoagulant therapy preceding surgery improves the prognosis and survival of patients. The sooner it starts, the better the results. When anticoagulants were administered during the first week, portal vein patency reached 60%. The same therapy started a week later was successful in 25% of cases [7, 20].
According to AASLD guidelines (2009), all patients with acute PVT should receive anticoagulation therapy for at least three months. Therapy begins with low molecular weight heparin followed by a transition to oral anticoagulants. The total duration of therapy is determined individually depending on the result achieved and the presence of thrombophilic disorders.
If septic pylephlebitis is suspected, antibacterial therapy is prescribed immediately.
The indication for emergency surgical intervention is the development of intestinal infarction: laparotomy and resection of the necrotic area of the intestine are performed [20].
Regarding the choice of optimal treatment tactics for chronic PVT ,
There is no consensus among clinicians. On the one hand, chronic PVT is often associated with prothrombotic disorders (the risk of developing intestinal ischemia and intestinal infarction increases); on the other hand, anticoagulant therapy is associated with a high risk of bleeding.
Taking into account concomitant portal hypertension, endoscopic examination is recommended in all patients with chronic PVT to exclude the presence of PVT. In 30% of patients with chronic PVT in the absence of cirrhosis, at least one episode of bleeding from varicose veins develops. In chronic PVT, recommendations for the treatment and prevention of varicose veins are the same as for portal hypertension due to cirrhosis (prophylactic administration of beta blockers for stage 2 varicose veins, endoscopic ligation for stage 3–4 varicose veins).
According to the AASLD recommendations (2009), anticoagulant therapy in patients with chronic PVT can be carried out in the absence of cirrhosis, while ensuring the prevention of bleeding from the esophageal tract and stomach and the presence of an increased risk of developing venous thrombosis.
For jaundice and other manifestations of portal biliopathy, biliary duct stenting can be performed [20].
It should be noted that the most controversial and difficult question remains about the treatment tactics for patients with PVT in the presence of cirrhosis. Do such patients need anticoagulant therapy? Arguments in favor of thrombolysis can include the following:
Chronic liver diseases can be considered as prothrombotic conditions, in which coagulation processes are activated in the liver, which in turn promotes the process of fibrogenesis. However, experimental studies have shown that anticoagulant therapy for chronic liver diseases can inhibit fibrogenesis processes;
in patients with cirrhosis, thrombosis of the intrahepatic sections of the hepatic (70%) and portal (30%) veins often develops, followed by liver atrophy and loss of some functioning hepatocytes;
with cirrhosis, there is a slowdown in blood flow in the portal vein, which creates additional conditions for the formation of a blood clot.
In addition, there is data on the restoration of blood flow in the portal vein without the development of gastrointestinal bleeding during anticoagulant therapy in this category of patients. However, these studies include a small number of observations, usually uncontrolled, and their results cannot be considered statistically reliable [21].
Is there an alternative to drug thrombolysis? Currently, invasive direct and indirect thrombolysis are actively discussed as an alternative.
With direct thrombolysis
, which is more technically complex, the anticoagulant is delivered directly to the site of thrombosis (portal or mesenteric vein) through a transjugular shunt or percutaneous transhepatic route, followed by thrombus suction, balloon dilatation, or stenting. Direct thrombolysis is carried out in acute PVT until a network of collateral vessels has formed.
With indirect thrombolysis
catheterization of the femoral or radial artery is performed, followed by delivery of an anticoagulant (for example, urokinase) into the superior mesenteric vein. Indirect thrombolysis can promote thrombus lysis, stimulate collateral angiogenesis and improve the clinical picture [22].
Please note: the above minimally invasive interventional radiology techniques can only be used in cases of acute and subacute thrombosis of the portal and superior mesenteric veins in the absence of signs of intestinal necrosis, perforation or peritonitis.
Direct thrombectomy
As an alternative to drug thrombolysis for PVT, it is not recommended due to the risk of rethrombosis and postoperative complications. However, thrombectomy when thrombosis lasts less than 30 days (via a percutaneous transhepatic approach) may have several advantages.
surgical decompression may be the treatment of choice.
- shunting, which is resorted to when treatment of complications of portal hypertension (bleeding from varicose veins) by medication or endoscopic methods (endoligation and sclerotherapy) is ineffective. At the same time, we must remember that in 37% of cases PVT is accompanied by thrombosis in the splenic and mesenteric veins. Consequently, this method in this case is considered non-radical. The advantages of shunting include the possibility of curing portal biliopathy and hypersplenism.
There are several options for bypass: splenorenal, mesocaval, ileorenal side-to-side, a shunt between the superior mesenteric and portal veins, TIPS [18].
PVT as a diagnostic and prognostic factor
Another important clinical point is the possibility of using the fact of PVT development as a prognostic criterion for various conditions. Thus, along with age and MELD scores, the development of PVT serves as an additional predictor of poor prognosis after liver transplantation.
Until recently, PVT was an absolute contraindication for liver transplantation. The first information about successful liver transplantation in patients with PVT appeared in 1985 [23]. Currently, PVT can be considered as an indication for transplantation if conservative or surgical treatments fail (similar to patients with encephalopathy, hypoxia and pulmonary artery hypertension).
Conclusion
For a long time, it was believed that portal vein thrombosis was a rare pathology. However, already in the 1970s. studies have demonstrated a significant incidence of PVT (1%) in the general population, which is likely due to improved diagnostic methods (CT, contrast-enhanced MRI). The clinical manifestations of PVT are extremely varied, ranging from an asymptomatic course in chronic thrombosis to ischemia and infarction of the intestine in acute thrombosis involving mesenteric vessels. In acute PVT, anticoagulant therapy is administered to prevent the spread of the thrombus to the mesenteric vessels and to recanalize the existing thrombus. An alternative to drug thrombolysis can be considered direct and indirect thrombolysis with the introduction of an anticoagulant directly into the portal or mesenteric vein. The issue of treatment of chronic thrombosis remains controversial: due to the lack of controlled studies, recommendations for chronic PVT coincide with those for the treatment of portal hypertension in patients with cirrhosis. In uncorrectable cases of portal biliopathy and hypersplenism, various types of bypass surgery are possible.
Disease prognosis
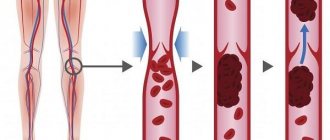
The outcome of thrombosis varies. Favorable ones include aseptic autolysis of a blood clot, which occurs under the influence of proteolytic enzymes of leukocytes. Small thrombi can be completely subjected to aseptic autolysis. More often, blood clots, especially large ones, are replaced by connective tissue, that is, they are organized. The ingrowth of connective tissue into a thrombus begins in the region of the head from the intimal side of the vessel, then the entire mass of the thrombus is replaced by connective tissue, in which cracks or channels lined with endothelium appear, the so-called. clot drainage. Later, the channels lined with endothelium turn into vessels containing blood; in such cases, they speak of vascularization of the thrombus, which often restores the patency of the blood vessel. However, the organization of a thrombus does not always end with its canalization and vascularization. Calcification of the thrombus and its petrification are possible; In this case, stones sometimes appear in the veins - phleboliths [3, 9, 10, 13].
Unfavorable outcomes of thrombosis include: • separation of a thrombus or part of it and transformation into a thromboembolus, which is a source of thromboembolism; • septic melting of a blood clot, which occurs when pyogenic bacteria enter the thrombotic masses, which leads to thrombobacterial embolism of the vessels of various organs and tissues (in sepsis). The prognosis for PVT is completely determined by the etiology of the disease. In adults with PVT, the 10-year survival rate is, according to various sources, 38–60% [9, 21]. Patients die mainly from complications of underlying diseases (cirrhosis, liver cancer). Mortality from bleeding from varicose veins in patients with PVT without cirrhosis does not exceed 5%, while in patients with cirrhosis this figure is 30–70% [9,21].
In conclusion, it should be noted that PVT is a serious disease that requires immediate diagnosis and intensive treatment in order to prevent complications, such as the formation of cavernous transformation and progression of PG. The survival of patients with portal thrombosis directly depends on the speed and thoroughness of diagnosis, the use of modern methods of instrumental and laboratory examination and visualization of thrombosis, and the assessment of concomitant thrombophilic conditions that increase the risk of complications. Early anticoagulant and antiplatelet therapy promotes reperfusion of thrombosed areas of the veins of the portal system and, despite the risk of gastrointestinal bleeding, is necessary for patients with PVT, as well as with diagnosed PG and cavernous transformation of VV.
Practical Basics
Portal vein thrombosis (PVT) is increasingly recognized by ultrasound. Decreased portal blood flow caused by liver parenchymal disease and abdominal sepsis (ie, infectious or ascending thrombophlebitis) are the main causes.
PVT is a common complication of liver cirrhosis, and its prevalence increases with the severity of liver disease, ranging from 1% in patients with compensated cirrhosis to 8–25% in liver transplant candidates.
Correct diagnosis and characterization of PVT are important for prognosis and further treatment. Portal vein thrombosis is a poor prognostic indicator, found at diagnosis in 10-40% of patients with hepatocellular carcinoma (HCC). Survival is approximately 2-4 months.
CEUS allows detailed visualization of the hepatic microvasculature, focal liver lesions, and portal vein thrombosis. Malignant thrombi have the same pattern of enhancement as the tumor from which they originated, including rapid arterial phase elevation and slow or weak washout in the portal vein.
Treatment of thrombophlebitis of superficial veins
The main therapeutic measures are reduced to elastic compression (elastic bandage or compression hosiery) and the prescription of medications.
Medicines used include phlebotropic drugs (detralex, phlebodia), antiplatelet agents (thrombo-ACC), and anti-inflammatory drugs (Voltaren). Lyoton-gel is applied topically.
All patients require venous ultrasound to exclude concomitant deep vein thrombosis and clarify the prevalence of superficial vein thrombophlebitis.
Causes of thrombosis in the superior vena cava system
The causes of thrombosis in the superior vena cava system are basically the same as other venous thrombosis. It may also develop as a complication of venous catheterization (cubital, subclavian catheter), sometimes arising as a result of prolonged compression or uncomfortable position of the upper limb (for example, during sleep).
The most common is thrombosis of the axillary or subclavian vein (Paget-Schrötter syndrome). Within 24 hours, swelling of the entire upper limb occurs with cushion-like swelling of the hand. There may be slight bursting pain. The color of the limb is unchanged or slightly cyanotic.