Classification of pathological condition
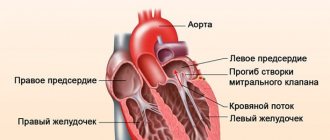
Mitral valve prolapse is a disease that can be congenital or acquired. In addition, the defect is classified into audible and silent. In the latter case, the disease is detected only during examination by a cardiologist.
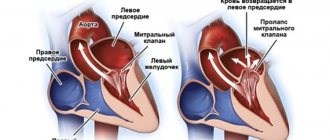
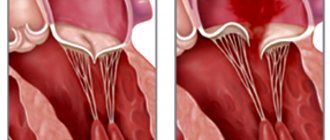
Classification according to the degree of protrusion of the atrioventricular valve leaflets into the atrium cavity:
- 1st degree – up to 0.5 cm;
- 2nd degree – from 0.5 to 0.9 cm;
- 3rd degree – more than 1 cm.
The disease is most often diagnosed at the age of 5-7 years. Due to the fact that modern examination methods are used today, such as echocardiography, the disease is diagnosed at an early age. According to studies, it was found that up to 15% of children suffer from the pathological condition in question.
Signs of mitral valve prolapse
Clinical manifestations depend on the degree of connective tissue dysplasia. The main symptom is heart rhythm disturbances - acceleration, tremors, freezing, interruptions. During physical activity, stress, and drinking coffee, extrasystole, a feeling of lack of air, shortness of breath, and tachycardia often occur. Some patients experience shortness of breath even with little activity.
Other possible signs:
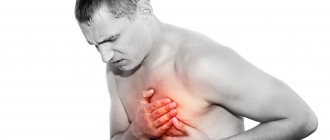
- chest pain;
- dyspnea;
- hyperventilation syndrome;
- vegetative crisis;
- increased sweating;
- anxiety;
- increased fatigue;
- headache;
- dizziness;
- feeling of a lump in the throat;
- decreased performance;
- irritability;
- mood swings;
- depression;
- presyncope and fainting;
- slight increase in body temperature.
Etiology of the disease
The main cause of the disease is dysgenesis of connective tissue, which is part of many organs and, in particular, the valve apparatus.
Underdevelopment of connective tissue can be caused for various reasons. These could be infections, intoxications and occupational hazards that affected the body of a pregnant woman and the fetus. There is no clear answer to what causes the disease.
The acquired form of the pathological disease occurs after an inflammatory disease, in particular infective endocarditis, rheumatism.
Causes and degrees of mitral valve prolapse
Pathology can be primary and secondary. In the first case, the cause is connective tissue dysplasia. It causes a change in the structure and length of the chords by which the valve flaps are attached to the muscles, or contributes to the appearance of additional chords.
Secondary prolapse accompanies or complicates other pathologies. Possible reasons:
- congenital heart defects;
- endocrine disorders;
- rheumatic diseases;
- connective tissue diseases;
- genetic syndromes.
Risk factors are valvular-ventricular disproportion, infective endocarditis, insufficient blood supply to the papillary muscles, hypertrophic cardiomyopathy, inflammatory damage to the valvular structures of the heart, myocarditis, hypertrophy of the ventricular wall, pericarditis, coronary heart disease, chest trauma, etc.
The pathology is divided into 3 degrees depending on how much the valve leaflets protrude:
- 3–5 mm – I degree;
- 6–9 mm – II degree;
- ≥ 10 mm – III degree.
The course of the disease is usually benign. In most patients, the disorder does not progress throughout their lives. There are known cases of a decrease in the severity of pathology with age. Complications are rare, but they are possible.
Symptoms
These include the so-called stigmas - signs of imperfection of the connective tissue framework: joint hypermobility, winged shoulder blades, myopia, Gothic (high) palate, malocclusion, flat feet, tall stature and asthenic physique, scoliosis, hernias, varicose veins. There is also a violation of the architectonics of the atrioventricular ring with displacement of the valves and additional chords in the valve apparatus.
Later, by the age of 7, additional symptoms appear: short-term stabbing pains in the heart area of a psycho-emotional nature, and not due to damage to the heart muscle, attacks of rapid heartbeat, interruptions.
Symptoms of vascular dystonia include dizziness, cephalgia in the morning, worsening after overwork, decreased performance and increased fatigue. Children are tearful, often have fears, depression, irritability, and poor sleep. In fact, this is a mass of microanomalies combined with vegetative-vascular instability.
A pediatrician or cardiologist listens to the murmur over the heart area and determines its nature - late systolic or in the form of a systolic click.
Yu.M. Belozerov, Sh.M. Magomedova, I.M. Osmanov Moscow Research Institute of Pediatrics and Pediatric Surgery of the Ministry of Health and Social Development of the Russian Federation
It has been established that mitral valve prolapse (MVP) accounts for 3-5% of the population in the structure of childhood cardiovascular diseases [1]. Recent studies have shown the genetic and phenotypic heterogeneity of MVP syndrome [2, 3]. With autosomal dominant inheritance, 3 genes of the syndrome were found, which are mapped on chromosomes 16p12.1, p11.2 and 13 [4]. Another locus was found on the X chromosome (Xq28) and causes a rare form of MVP, which is designated “X-linked myxomatous valvular dystrophy” [5]. C. Yosefy, A. Ben Barak [6] identified polymorphism of the fibrillin 1 gene in exon 15 TT and exon 27 GG. This genetic heterogeneity of the syndrome causes the occurrence of various forms of primary MVP, as well as a wide range of clinical manifestations depending on age [7]. Various mutations of the listed genes lead to defective functioning of connective tissue, especially fibrillin, elastin, collagen types I and III [8]. Impaired fibrillogenesis of the extracellular matrix causes myxomatous infiltration of the mitral valve leaflets, deficiency of fibroelastic fibers, changes in collagen, and accumulation of mucopolysaccharides. Based on the “continuum of transition states,” Russian authors identify the concept of undifferentiated connective tissue dysplasia (UCTD) [9, 10], which underlies PMC. According to the Framingham Heart Study of the American College of Cardiology (ACC) and the American Heart Association, the diagnosis of MVP can only be made if two diagnostic signs are present: auscultatory and echocardiographic [11].
Material and methods All examined patients were divided into the following groups: 1st group MVP - with undifferentiated connective tissue dysplasia (UCTD) - 340 children and adolescents (this group also included primary and familial MVP with connective tissue dysplasia - DCT); Group 2 MVP - with differentiated connective tissue dysplasia (CTD) - 65 children and adolescents (this group included patients with Marfan and Ehlers-Danlos syndrome). The control group consisted of 200 children and adolescents of the same age. A scoring of the phenotypic manifestations of connective tissue dysplasia was made for group 1 - three subgroups (340 patients) were identified. A study of the elastic properties of the aortic wall was carried out in patients from both groups (64 patients). Study of the exhaustion of the autonomic nervous system - in 200 patients from all three groups, including the control.
Results and discussion According to the anamnesis, many children with MVP, both undifferentiated and differentiated CTD, were born to mothers with an unfavorable course of pregnancy and childbirth. Early and late gestosis were observed significantly more often in mothers who had children with MVP against the background of DSTD (56.9 compared to 25.8% with NSTD; pComplaints from patients with MVP with undifferentiated and differentiated DSTD were realized in a symptom complex of chronic psycho-emotional stress, the frequency of which was in 6 times higher than that of the control group of healthy children. Chronic psycho-emotional stress leads to neurocirculatory instability. Neurocirculatory instability (the term adopted by the European Association of Cardiology in 1995) is understood as a violation of the autonomous regulation of vascular tone in adolescents of the pre- and pubertal period [12 ] Unlike neurocirculatory dystonia, neurocirculatory instability is not accompanied by a persistent deviation of vascular tone - arterial hypo- and hypertension.Rises in blood pressure with neurocirculatory instability are variable, arterial hypertension can be replaced by arterial hypotension. Such changes in blood pressure can lead to somatic disorders and the formation of a specific somatotype. Significantly more often in children with DSTD compared to NSTD (52.3% and 20.2%, p In the examined children with MVP against the background of DSTD, the sensation of heartbeat can be associated with various reasons:
• disproportionate tachycardia during emotional or physical stress; • increased pulsation of the dilated aorta; • thoradiaphragmatic changes; • heart rhythm disturbances; • neuropsychiatric symptoms (panic attacks, nervousness, vegetative-vascular dystonia).
A significant increase in the frequency of cardialgia was observed in children and adolescents with MVP with undifferentiated and differentiated CTD. Pain in the heart area was characterized as “stabbing”, “pressing”, “aching” and was felt in the left half of the chest without any irradiation. In most children they lasted for 5-20 minutes, usually occurred when running, quickly climbing stairs, due to emotional stress and were usually accompanied by vegetative disorders: unstable mood, cold extremities, “chilliness”, palpitations, sweating, passed spontaneously and after taking sedatives. The absence of ischemic changes in the myocardium, according to a comprehensive examination, allows cardialgia to be regarded as a manifestation of sympathalgia associated with the psycho-emotional characteristics of children with MVP (“pain in the heart is inseparable from personality”). According to the literature, pain in the heart area in children and adolescents with MVP and CTD may be due to the following reasons:
• excessive tension of the chordae, leading to overstretching of the papillary muscles; • microembolism of the coronary arteries due to increased platelet aggregation and fibrinous deposits located in the angle between the left atrium and the posterior mitral leaflet; • disproportionate tachycardia during physical and/or emotional stress; • hyperadrenergic status, which increases myocardial oxygen demand; • spasm of the coronary arteries.
Fainting conditions were observed only with MVP against the background of DSTD and were caused by an orthostatic drop in blood pressure. Recently, the issue of disruption of neurovegetative regulation of cardiac activity and vascular tone has been discussed. Thus, many authors note orthostatic hypotension in Marfan syndrome [13], often accompanied by headaches [14]. The genesis of the resulting fainting is not fully understood. Our data confirm the presence of orthostatic instability in children and adolescents with DSTD and MVP, and fainting has a vasodepressor origin. The auscultatory picture with MVP against the background of undifferentiated and differentiated DST was almost the same. Most often, the auscultatory phenomenon of a combination of clicks with late systolic murmur was determined (53.5 and 42.9%, respectively), then late systolic or holosystolic murmur (13.3 and 30.8%, respectively, p There is a certain parallelism in the severity of leaflet prolapse and sound phenomena. With isolated clicks, the deflection of the valves is usually small, with isolated late-systolic and holosystolic murmurs it is significant. It is known that the general biological significance of growth is to achieve a level of development of the organism that is necessary for its reproduction. In humans, the final growth program is to achieve not only reproductive, but intellectual and social perfection [15].A high level of stigmata of connective tissue development was detected in children and adolescents with CTD, and we did not obtain significant differences in the subgroups with differentiated and undifferentiated dysplasia, with the exception of those disorders that are characteristic of a specific molecular genetic disease (Marfan disease - fibrillin-1 gene defect – 134797.0001, which is mapped to chromosome 15q21.1) or Ehlers-Danlos syndrome caused by mutations in the collagen alpha 1(V) gene (COL5A1; 120215), collagen alpha 2 A(V) (COL5A2; 120190) or collagen alpha 1(I) (COL1A1; 120150). On the other hand, the high frequency of stigmata of connective tissue dysembryogenesis in DST confirms the fact that, along with the genetic mechanisms of the disease, external factors are of great importance, which can cause significant individual deviations, clinical polymorphism of DST. The scoring of the phenotypic manifestations of connective tissue dysplasia in NSTD indicates a wide range of the studied parameters in this group of patients with MVP. In this regard, based on multifactor analysis, including anamnestic and genealogical data, as well as examination results, we identified three subgroups of children and adolescents with MVP against the background of NSTD:
1st – MVP with moderate manifestations of NSTD (score from 23 to 49 – 82 patients); 2nd – MVP with pronounced manifestations of NSTD (score from 50 to 75 – 196 patients); 3rd – MVP with pronounced manifestations of NSTD (score from 76 to 90 – 62 patients).
On physical examination, the boundaries of the heart in all examined patients corresponded to normal values, with the exception of children with chest deformities, when there was a shift (usually to the left) of the percussion boundaries of the absolute and relative dullness of the cardiac shadow. Quite often, in children with MVP against the background of NSTD, dilatation of the pulmonary artery was detected. It should be noted that X-ray examination allows to detect dilatation of the pulmonary artery (PA) and confirm its severity with a Moore index of more than 40%, but does not reveal the true cause of PA dilatation. We compared the Moore index with the level of stigmata of disembryogenesis. The Moore index was higher, the more phenotypic signs of connective tissue dysembryogenesis were determined. This confirms the connection between pulmonary artery dilatation and the inferiority of the connective tissue matrix. The main electrocardiographic abnormalities found in NSTD and DSTD occur in 30% of cases and include changes in the terminal part of the ventricular complex, cardiac rhythm and conduction disturbances, and prolongation of the QT interval. Repolarization anomalies are detected both during a standard ECG and much more often during 24-hour electrocardiogram monitoring [16]. This fact indicates the presence of hidden myocardial instability in children with MVP syndrome against the background of both differentiated and undifferentiated DST. The appearance of repolarization changes in orthoposition can be explained by an increase in the tension of the papillary muscles due to the resulting tachycardia, a decrease in the volume of the left ventricle and an increase in the depth of leaflet prolapse. If previously the cause of such ST-T changes on the ECG during MVP was associated with ischemic impairment of the coronary circulation or was considered as a combined manifestation of a dysplastic process in the heart, now most authors see the cause of repolarization disorders during MVP as hypersympathicotonia. This statement is justified by the fact that ST-T changes in MVP are variable in nature and completely disappear during a pharmacological stress test with a b-blocker. A few publications report the occurrence of myocardial ischemia in MVP syndrome in children [17]. The genesis of transient ischemia in such patients may be due to congenital anomalies of the coronary vessels. We identified myocardial ischemia during physical activity, if the process of repolarization of ischemic genesis deteriorated, and was detected in a few patients (2 patients with MVP and NSTD and 3 patients with DSTD). We conducted a study of minor anomalies in the development of cardiac structures in children with MVP and CTD. Most of the microanomalies are related to the connective tissue structures of the heart. Some minor anomalies, such as impaired distribution of the chordae, may be directly related to the MVP syndrome, being a causative factor. Other anomalies, such as dilatation of the great vessels, coronary sinus, etc., reflect the inferiority of connective tissue structures. Of particular importance are abnormally attached tendon chords of the subvalvular apparatus. A number of authors consider them to be the cause of mitral valve prolapse [18]. With connective tissue dysplasia, not only the valve apparatus of the heart is affected, but also the great vessels. In this regard, we undertook a study of the elastic properties of the aortic wall in adolescents with MVP using the method of E. Michelfelder et al. [19]. The distensibility index and stiffness of the aorta were determined. The study was conducted in 64 adolescents aged 15-18 years (average age 16.4±0.7), divided into 2 subgroups: 1st subgroup - 48 patients (33 girls and 15 boys) with MVP due to NSTD; 2nd subgroup – 16 (10 girls, 6 boys) patients with MVP and DSTD. A significant increase in the aortic distensibility index was established (aortic distensibility: control 0.0021±0.005 mmHg-1, MVP and NSTD - 0.0035±0.007 mmHg-1, MVP and DSTD - 0.0038±0.006 mmHg-1 (pCharacteristic vegetative changes were established with MVP against the background of NSTD. With an increase in the degree of DST, there was a significant increase in vagotonic signs, which may be associated with a compensatory reaction to the initial sympathicotonia. The study of the state of the autonomic nervous system made it possible to establish a characteristic autonomic pattern in DSTD, when the initial sympathicotonia is combined with asympathicotonic reactivity. This this fact indicates a low adaptive ability and rapid "exhaustion" of the sympathetic part of the ANS. Many children with MVP, mainly in adolescence, exhibit psycho-emotional disorders, represented by depressive and asthenic symptom complexes. In order to assess the psychological portrait of the individual, we used the abbreviated multifactor personality questionnaire - SMOL . When determining the psychological profile (200 adolescents were examined) of an individual by testing the SMOL, we obtained a number of patterns. Indicators of the average profile of SMOL in adolescents of the control group did not go beyond the conventionally accepted norm: >70 and Maximum characterological changes were found in adolescents with MVP against the background of NSTD. Noteworthy were the high values on the second and seventh scales, exceeding 70 points, which reflected pronounced psychological maladaptation, as well as profile peaks on the third scale, emphasizing exalted behavior and emotional lability. In addition, intergroup differences were obtained for the indicators of this scale between the groups with NSTD and DSTD. Noteworthy is also the peak of the profile on the first scale (67.5 points), approaching the conventional border of mental norm/pathology - 70 points. This indicates a significantly pronounced tendency towards aggravation, “going into illness” among adolescents in this group, which is combined with pronounced emotional lability. In the group of adolescents with MVP and DSTD, significant increases in the profile were found on the second, third and seventh scales relative to the comparison group, which reflected a gloomy mood background, increased anxiety combined with a tendency to hysterical reactions. A decrease in the quality of life in adolescents with various types of MVP has been revealed, affecting both mental and physical health. The degree of reduction in quality of life and the number of scales affected depends on the severity of connective tissue dysplasia. Adolescents with both MVP and DSTD and MVP and NSTD were characterized by a decrease in the quality of life on almost all scales studied. In scales characterizing physical functioning, the lowest scores were observed in the group of people with MVP and DSTD. As for the mental component of health, the lowest values were observed in the group of adolescents with MVP and NSTD, although in patients with MVP and DSTD they were also significantly lower than control values. Thus, we can conclude that the degree of connective tissue disorders is the leading factor determining the quality of life of a teenager. The management tactics for children with MVP vary depending on the degree of connective tissue dysplasia, the severity of leaflet prolapse, and the nature of autonomic and cardiovascular changes [20]. We noted a positive result in the treatment of children with MVP and DST with carnitene and magnesium preparations. In children and adolescents with MVP and CTD, in most cases, there is a deficiency of carnitine, which is confirmed by chronic fatigue syndrome. A test with carnitene is an indicator of the insufficiency of trophotropic mechanisms in the regulation of cardiac activity. A positive response to carnitene is indicated by changes in HRV in the form of an increase in VLF and a decrease in HF. All children with mitral valve prolapse are prescribed carnitene to improve energy processes in the heart muscle. There is no doubt that energy-tropic therapy with carnitene (based on the examination of 30 children) is an important element in the correction of metabolic disorders in the heart muscle in patients with MVP and CTD. On the other hand, the positive effect of the therapy confirms the large role of secondary mitochondrial failure in the pathogenesis of connective tissue dysplasia. We also conducted a study of the effect of the drug Magnerot® on the structure of the mitral valve leaflets in MVP associated with NSTD. In the initial values for MVP, a significantly greater thickness of the leaflets in boys was revealed (t = 5.11 p After a 6-month course of therapy with Magnerot® in patients with MVP and NSTD, a significant improvement in objective and subjective symptoms was found with a complete or almost complete reduction in the manifestations of the disease by more than in half of the patients. During treatment, there was a decrease in the severity of vegetative dystonia syndrome, vascular, hemorrhagic and psychopathological disorders, heart rhythm disturbances, blood pressure levels, as well as an improvement in the quality of life of patients. In addition, during treatment, the severity of morphological markers of connective tissue dysplasia significantly decreased [ 10]. Thus, the data obtained indirectly indicate that in the basis of dysplastic development of connective tissue structures, not only genetic factors play a great role, but also pathological “maturation”, differentiation of connective tissue with impaired fetal development. It should be noted that the development and the maturation of the mitral apparatus, connective tissue structures and the autonomic nervous system occurs synchronously at the same time of embryogenesis and postnatal ontogenesis.
References 1. Sakamoto S. Mitral valve prolapse. Nippon Rinsho. July 2005; 63 (7):1195-2000. 2. Yosefy C, Levine RA, Picard MH et al. Pseudodyskinesis of the inferior left ventricular wall: recognizing an echocardiographic mimic of myocardial infarction. J Am Soc Echocardiogr. 2007 Dec; 20 (12): 1374-9. 3. Martínez-Sellés M., García-Fernández M.A., Larios E. et al. Etiology and short-term prognosis of severe mitral regurgitation. Int J Cardiovascular Imaging. Feb 2009; 25 (2): 121-6. 4. Levine RA, Slaugenhaupt SA Molecular genetics of mitral valve prolapse. Curr Opin Cardiol. May 2007; 22 (3): 171-5. 5. Grau JB, Pirelli L., Yu PJ, Galloway AC, Ostrer H. The genetics of mitral valve prolapse. Clin Genet. 2007 Oct; 72 (4): 288-95. 6. Yosefy C., Ben Barak A. Floppy mitral valve/mitral valve prolapse and genetics. J Heart Valve Dis. 2007 Nov; 16 (6): 590-5. 7. Romanelli P., Romanelli R., Rongioletti F. et al. Clinical significance of cutaneous proteoglycan (mucin) infiltration in patients with mitral valve prolapse. J Am Acad Dermatol. July 2008; 59 (1): 168-9. 8. Gupta-Malhotra M., Dave A., Sturhan BC et al. Prevalence of undiagnosed congenital cardiac defects in older children. Cardiol Young. 2008 Aug; 18 (4): 392-6. 9. Martynov A.I., Stepura O.B., Ostroumova O.D. etc. Mitral valve prolapse. Part I. Phenotypic features and clinical manifestations. Cardiology. 1998; 1: 72-80. 10. Melnik O.O., Ostroumova O.D., Stepura O.B. Mitral valve prolapse - normal or pathological? 2002; 10:28:1314-28. 11. Freed LA, Levy D, Levine RA et al. Prevalence and clinical outcome of mitral valve prolapse. N.Engl. J. Med. 1999, 341(1): 1-7. 12. Mochizuki Y., Okutani M., Donfeng Y. et al. Limited reproducibility of circadian variation in blood pressure dippers and nondippers. Am J Hypertens. 1998 Apr; 11 (4 Pt 1): 403-9. 13. van Dijk N., Boer MC, Mulder BJ, van Montfrans GA, Wieling W. Is fatigue in Marfan syndrome related to orthostatic intolerance? Clin Auton Res. 2008 Aug; 18 (4): 187-93. 14. Rosser T., Finkel J., Vezina G., Majd M. Postural headache in a child with Marfan syndrome: case report and review of the literature. J Child Neurol. 2005 Feb; 20 (2): 153-5. 15. Veltishchev Yu.E. Child growth: patterns, normal variations, somatotypes, disorders and their correction. 2000; 66. 16. Digeos-Hasnier S., Copie X., Paziaud O., Abergel E., Guize L. et al. Abnormalities of ventricular repolarization in mitral valve prolapse. Ann Noninvasive Electrocardiol. July 2005; 10 (3): 297-304. 17. Guthmann JP, Rossignol AM, Wolf JE, Azoulay A., Bost M. Transient myocardial ischemia and isolated congenital mitral valve prolapse in an infant. Arch Mal Coeur Vaiss. May 1991; 84 (5): 735-8. 18. Boon R., Hazekamp M., Hoohenkerk G., Rijlaarsdam M., Schoof P. et al. Artificial chordae for pediatric mitral and tricuspid valve repair. Eur J Cardiothorac Surg. July 2007; 32 (1): 143-8. 19. Michelfelder EC, Khoury P., Witt SA, Glascock BJ, Kimball TR Noncircumferential myofiber function: impact on early diastolic filling in children. J Am Soc Echocardiogr. 2001 Nov; 14 (11): 1065-9. 20. Scordo KA Medication use and symptoms in individuals with mitral valve prolapse syndrome. Clin Nurs Res. Feb 2007; 16 (1): 58-71.
Diagnostics
The main diagnostic method is conventional auscultation of the heart (listening). When prolapse occurs, the doctor hears specific noises and clicks.
An ECG is not advisable because it does not show any abnormalities. The key test is echocardiography. If necessary, angiocardiography, left-sided ventriculography, and radionuclide testing are additionally performed.
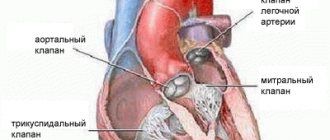
Differential diagnosis is carried out with aneurysm of the interatrial septum, mitral valve insufficiency, acquired heart defects.