Medicine/8. Morphology
Ph.D. Rybakov A.G., Ph.D. Loshkarev I.A., Ph.D. Machinsky P.A.,
Ph.D. Tishkov S.V.
Mordovian State University named after. N.P. Ogareva, Russia
Variant anatomy of the anterior communicating artery of the circle of Willis
The circle of Willis is an arterial anastomosis located at the base of the brain in the subarachnoid space between the anterior part of the optic chiasm and the pons. The circle of Willis has two parts: front and back. The anterior part is formed by the initial sections of the two anterior cerebral arteries, which arise from the internal carotid arteries and are connected by the anterior communicating artery. The posterior part is formed by two posterior cerebral arteries, which, with the help of paired posterior communicating arteries, connect to the internal carotid artery on their side. Thus, the branches of the internal carotid and vertebral arteries take part in the formation of the circle of Willis. The connecting arteries serve as a connecting link between the sections of the circle of Willis.
Along with the typical structure of the arteries of the base of the brain, there are atypical varieties of the circle of Willis (1, 2, 8, 9). Variants and anomalies of the vessels of the arterial circle of the brain are important in neurological and neurosurgical practice (3, 4, 5, 7). Among the various variants of the structure of the arteries of the base of the brain, the most hemodynamically unfavorable are aplasia and hypoplasia of the communicating arteries. In this case, anatomical and functional disconnection of the circle of Willis occurs, and when cerebral circulation is impaired, extensive foci of ischemic brain damage develop (1, 6).
The purpose of our study was to study the structural options of the anterior communicating arteries of the circle of Willis.
The study was carried out on 124 preparations of the arteries of the base of the human brain. The vessels of the arterial circle were isolated by dissection.
Research results. The typical structure of the anterior communicating artery was found in 94 cases (75.8%), various variants of the structure of the anterior communicating artery were observed in 29 cases (23.4%), and in 1 case (0.8%) we found an aneurysm of the anterior communicating artery.
In typical cases, the anterior communicating artery is a stem 2-4 mm long, 1.5-2.5 mm in diameter, which is located between the initial sections of the anterior cerebral arteries (Fig. 1).
Rice. 1.
Typical structure of the anterior communicating artery (indicated by an arrow)
Among the variants, the most common was the median artery of the corpus callosum, which was found in 10 cases (8.0%). The median artery of the corpus callosum originated from the anterior communicating artery and was directed between the right and left anterior cerebral arteries along the corpus callosum (Fig. 2).
Rice. 2.
Median artery of the corpus callosum (indicated by arrow)
In 8 cases (6.5%), a double anterior communicating artery was observed, which in the form of two parallel trunks connected the right and left anterior cerebral arteries (Fig. 3a). In 3 cases (2.4%) partial bifurcation of the anterior communicating artery was found. In this case, the artery began with one stem from the anterior cerebral artery on one side, and then forked fork-shaped and connected with the anterior cerebral artery of the opposite side (Fig. 3b).
Rice. 3 A.
Double anterior communicating artery (indicated by arrow)
Rice. 3 B.
Partial bifurcation of the anterior communicating artery (indicated by an arrow)
the median artery of the corpus callosum is also visible
In 1 case (0.8%) a triple anterior communicating artery was observed (Fig. 4a) and in 1 case (0.8%) a vascular network was found at the site of the anterior communicating artery (Fig. 4b).
In 4 cases (3.2%), hypoplasia of the anterior communicating artery was noted, which was represented by a thread-like branch located between the right and left anterior cerebral arteries (Fig. 5a). In 5 cases (4.0%), the anterior communicating artery was absent, and the anterior cerebral arteries were connected to each other using a side-to-side anastomosis (Fig. 5b). The specified connection was located vertically over a distance of 4-5 mm.
In 1 case (0.8%), an aneurysm of the anterior communicating artery was found, which had a round shape and a diameter of about 2.5 mm (Fig. 6).
Rice. 4 A.
Triple anterior communicating artery (indicated by arrow)
Rice. 4 B.
Anterior communicating artery in the form of a vascular network (indicated by an arrow)
Rice. 5A.
Hypoplasia and partial bifurcation of the anterior communicating artery
(indicated by arrow)
Rice. 5 B.
The anterior communicating artery is absent, the anterior cerebral arteries are connected to each other using a side-to-side anastomosis (indicated by an arrow)
Rice. 6.
Aneurysm of the anterior communicating artery (indicated by arrow)
Literature
1. Belenkaya R.M. Stroke and variants of the cerebral arteries. M.: Medicine, 1979. – 176 p.
2. Gladilin Yu.A. Anatomical features of the internal carotid arteries and the arterial circle of the cerebrum. – Saratov: Publishing House of Saratov Medical University, 2008. – 242 p.
3. Krylov V.V., Tkachev V.V., Dobrovolsky G.F. Microsurgery of aneurysms of the polygon of Willis. – M.: Antidor, 2004. – 160 p.
4. Pucillo M.V., Vinokurov A.G., Belov A.I. Neurosurgical anatomy. Ed. A.N. Konovalova. – M.: Antidor, 2002. – 206 p.
5. Alawad AH, Hussein MA, Hassan MA Morphology and normal variations of the cerebral arterial circle of Willis in Khartoum Diagnostic Center // Khartoum Medical Journal. – 2009. – Vol. 2, No. 2. – P. 215-219.
6. De Silva K., Silva R. et al. Prevalence of typical circle of Willis and the variation in the anterior communicating artery: a study of a Sri Lankan population // Ann. Indian. Acad. Neurol. –2009. – Vol. 12, No. 3. – P. 157-161.
7. Gurdal E., Cakmak O. et al. Two variations of the anterior communicating artery: a clinical reminder // Neuroanatomy. – 2004. – Vol. 3. – P. 32-34.
8. Kapoor K., Singh B., Dewan LI Variations in the configuration of the circle of Willis // Anat. Sci. Int. – 2008. – Vol. 83, No. 2. – P. 96-106.
9. Nayak SB, Somayaji SN, Soumya KV Variant arteries at the base of the brain //
International Journal of Anatomical Variations. – 2009. – Vol. 2. – P. 60-61.
Medical Internet conferences
O.A. Fomkin - Saratov State Medical University named after. IN AND. Razumovsky Ministry of Health of Russia, assistant at the Department of Human Anatomy, Candidate of Medical Sciences; V.N. Nikolenko - State Budgetary Educational Institution of Higher Professional Education First Moscow State Medical University named after. THEM. Sechenov Ministry of Health of Russia, Vice-Rector for Scientific and Innovation Activities, Professor of the Department of Human Anatomy, Director of the Research Institute of Molecular Medicine, Professor, Doctor of Medical Sciences; Yu.A. Gladilin – State Budgetary Educational Institution of Higher Professional Education Saratov State University named after. IN AND. Razumovsky Ministry of Health of Russia, Associate Professor of the Department of Human Anatomy, Doctor of Medical Sciences.
Introduction. Variability as a general biological phenomenon does not lose its relevance and deserves the attention of many scientists [1, 2]. Variability shows the plasticity of living systems and is associated with the implementation of the adaptive strategy of a natural population. The study of variability makes it possible to judge the interaction of the genotype with environmental factors during ontogenesis [3].
In response to the demands of clinical medicine, at present, detailed information is required not so much on the typical structure or average anatomical norm of an organ, but on the entire spectrum of its individual, typical and combined variability [2]. This also applies to the arterial vessels of the brain.
The subject of this study is the posterior communicating artery (PCA). As a branch of the cerebral part of the internal carotid artery, it participates in the formation of the arterial (Circle of Willis) circle of the brain. It has been proven that blood through this artery can flow in both directions [4]. In this regard, the PCA plays an important role in the implementation of compensatory collateral blood flow between the systems of the internal carotid and vertebral arteries.
The structure of the posterior communicating arteries varies greatly. Compared to other cerebral arteries, they have a small diameter and almost a pinpoint lumen [5-7].
Purpose of the study: to determine the variants of the posterior communicating artery (PCA) of adults depending on the individual and combined variability of its morphometric parameters.
Materials and methods . The study material was PCA obtained from autopsies of 115 corpses of people aged 21 to 84 years who died for reasons not related to acute or chronic cerebrovascular pathology. A total of 230 artery samples were examined. To study the morphology of the artery, transverse millimeter sections were made using a razor. Then the sections were placed in a Petri dish with saline solution and the outer diameter and wall thickness of the artery were measured under a microscope with an accuracy of 0.01 mm. The diameter of the artery lumen is presented in the study as the difference between the outer diameter and twice the thickness of the artery wall.
The obtained data were processed by the variation-statistical method using the Statistica-6 application package and Microsoft Exsel Windows-XP. To check the presence of a normal distribution, the Kolmogorov-Smirnov test was used. The distribution of parameters in the studied sample did not differ from normal. In this regard, for all parameters, the minimum and maximum values, the arithmetic mean (M), the error of the arithmetic mean (m), the standard deviation (s), and the coefficient of variation (Cv) were determined. To assess the reliability of differences between series, a parametric test (Student's test) was used. In this case, the differences were considered significant at a 95% probability threshold (p < 0.05). When studying individual variability, like most researchers dealing with the range of anatomical norms, we took the variation interval M ± σ as the average value of a trait. Since statistically significant gender differences in the length, wall thickness and lumen diameter of the PCA were found [6], variations in these parameters were calculated separately for men and women.
Results. The average values of the morphometric parameters of the PCA (230 samples), excluding gender, age and cerebral hemisphere, were: 1) length 12.26±0.19 mm (A=5.30-20.10 mm; s=2.89 mm; Сv=23.6%); 2) outer diameter – 1.33±0.02 mm (A=0.80-2.10 mm; s=0.26 mm; Cv=19.2%); 3) wall thickness – 0.23±0.01 mm (A=0.12-0.40 mm; s=0.06 mm; Cv=26.2%); 4) lumen diameter – 0.88±0.02 mm (A=0.46-1.46 mm; s=0.25 mm; Cv=28.0%).
Significant variability in the morphometric parameters of the PCA made it possible to identify groups of variants of their values (Table 1).
According to the length of the PCA, they were divided into: short - length less than 9.89 mm in men and less than 8.80 mm in women; medium in length - with a length from 9.90 to 15.72 mm for men and from 8.81 to 11.51 for women; long – with a length of more than 15.73 mm in men and more than 11.52 mm in women. The average age of subjects with short PCAs was 1.2 times greater than that of people with long arteries – 51.3±2.9 years and 43.0±2.6 years, respectively (p=0.039). The quantitative ratio of men and women in the group of subjects with short arteries is 39.5 and 60.5%; in the group of subjects with long arteries – 71.9 and 28.1%.
Based on the size of the outer diameter, SSAs are: thin - diameter less than 1.06 mm; medium diameter (medium wide) - the diameter ranges from 1.07 to 1.59 mm and wide - with a diameter of more than 1.60 mm. Subjects with wide PCA were on average 1.2 times older than people with thin arteries - 55.0±3.0 years and 45.5±2.1 years, respectively (p=0.011). The quantitative ratio of men and women in the group of subjects with thin arteries is 59.0 and 41.0%; in the group of subjects with wide arteries, the representation of men and women is equal – 50% each.
Based on the wall thickness, the PCA was divided into: thin-walled - wall thickness less than 0.17 mm in men and less than 0.14 mm in women; medium in thickness - with a wall thickness from 0.18 to 0.30 mm for men and from 0.15 to 0.25 mm for women; thick-walled - with a wall thickness of more than 0.31 mm in men and more than 0.26 mm in women. The average age of subjects with thick-walled PCAs was 1.7 times greater than that of people with thin-walled arteries - 69.2±2.3 years and 40.7±2.5 years, respectively (p=1·10-6). The quantitative ratio of men and women in the group of subjects with thin-walled arteries was 25.7 and 74.3%; in the group of those studied with thick-walled arteries – 78.9 and 21.1%.
Depending on the size of the lumen diameter, we have identified PSA: with a narrow lumen - the lumen diameter is less than 0.58 mm in men and less than 0.68 mm in women; with an average lumen - the diameter of the lumen varies from 0.59 to 1.07 mm in men and from 0.69 to 1.19 mm in women; with a wide lumen - the lumen diameter exceeds 1.08 mm in men and 1.20 mm in women. The age of subjects with a narrow and wide lumen of the PCA did not differ significantly - 51.2 ± 2.5 years and 45.6 ± 3.1 years, respectively (p = 0.163). The quantitative ratio of men and women in the group of subjects with a narrow lumen of the PCA was 75.6 and 24.4%; in the group of those studied with a wide lumen of the PCA – 43.6% and 56.4%.
It was noted that approximately 71.9% of all thin ACAs had a narrow lumen, and 28.2% had an average lumen. At the same time, thin PCAs had thin wall thickness in 23.1% of cases, thick wall thickness in 5.2% of cases, and medium wall thickness in 71.8% of cases (Table 2).
Medium-wide arteries in 87.1% of cases were characterized by an average lumen diameter; in 7.7% of cases, such PCAs had a narrow, and in 5.2%, a wide lumen diameter. Medium-wide arteries, as a rule, had an average wall thickness (67.1% of observations); thin- and thick-walled PCA were also found in this group – in 12.9 and 20% of cases, respectively.
Wide PCAs had a wide lumen diameter in 86.1% of cases and an average lumen diameter in 13.9% of cases. Moreover, in 13.9% of cases they were thin- or thick-walled, and in the remaining 72.2% they were characterized by an average wall thickness.
The combined variability of the morphometric parameters of the PCA made it possible to identify 18 of its types. The most common are medium-wide ACAs with an average wall thickness and an average lumen (43.0%). Rare types (frequency of occurrence less than 1%) include thin PCA (with an average wall thickness and lumen diameter, with a thin wall and a narrow lumen, a thick wall and a narrow lumen, a thick wall and an average lumen); ASA with a medium outer diameter, a thick wall and a wide lumen; wide PCA with a thick wall and medium lumen (Fig. 1). Thin-walled SSAs based on our material never had a wide lumen diameter.
Rice. 1.Frequency of occurrence of types of PSA,% ( OD – outer diameter , TS – wall thickness, DP – lumen diameter ): 1 – average for ND, TS and DP; 2 – thin with medium TS and narrow DP; 3 – wide with medium TC and wide DP; 4 – average in ND, with a thick wall and average DP; 5 – average according to ND with a thin wall and average DP; 6 – thin with a thin wall and medium DP; 7 – average according to ND with a thick wall and narrow DP; 8 – average according to ND, with a thin wall and wide DP; 9 – average for ND, vehicles with narrow DP; 10 – wide with a thin wall and wide DP; 11 – wide with average wall thickness and average DP; 12 – wide with a thick wall and wide opening; 13 – thin with medium TC and medium clearance; 14 – average in ND, with a thick wall and wide DP; 15 – thin with a thin wall and narrow DP; 16 – thin with a thick wall and narrow DP; 17 – thin with a thick wall and medium DP; 18 – wide with a thick wall and medium DP.
Discussion. The vast majority of works concerning PCA are devoted to variants of its development: aplasia, hypoplasia, the presence of a vascular network at the site of the artery, etc. [5, 8, 9]. We presented information on the individual typological variability of the morphometric parameters of the PCA for the first time.
As a result of the study, it was revealed that the morphological parameters of the PCA (length, outer and inner diameter, wall thickness) are characterized by significant individual variability. The coefficient of variation of the studied parameters varies from 19.2% (outer diameter) to 28.0% (lumen diameter). For each of the parameters, we have identified 3 groups of arteries: I – with a value of the attribute less than average (<М-σ); II – with the average value of the trait (M±σ); III – with a trait value greater than average (>M+σ). No clear pattern was found in the predominance of men or women in the extreme groups of variability (groups I and II). Thus, among subjects with short, thin-walled arteries and arteries with a wide lumen, women predominate, and among subjects with long, thin, thick-walled arteries and arteries with a narrow lumen, men predominate. In the group of those studied with wide ACAs, the percentage representation of men and women is the same.
The average age of subjects whose PMA, based on the size of their outer diameter, wall thickness and lumen diameter, belongs to group III, is statistically significantly 1.2-1.7 times greater than that of people with PMA belonging to group I variability. The average age of men and women with short PCA (group I), on the contrary, is 1.2 times greater than that of people with long PCA (group III). The age of subjects with narrow or wide lumen PCA did not differ significantly.
Conclusion. Thus, the analysis of individual variability in the length, outer and inner diameters and wall thickness of the PCA made it possible to identify 3 groups of artery variants for each of the parameters: with an average value of the attribute, with a value of the attribute less and more than the average. The combined variability of the morphometric parameters of the PCA made it possible to identify 18 of its types.
The data obtained will complement and organize the existing information on the dimensional characteristics of the PCA, which is important for a better understanding of the area of neuromorphology under study, and can also be useful in modeling blood flow in the arterial circle of the brain.
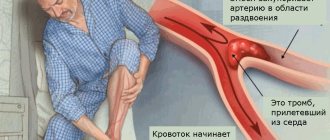
Variations in the development of cerebral arteries and epilepsy
The prevailing ideas about the mechanisms of development of cerebral ischemia imply the occurrence of a discrepancy between the available blood supply and the needs of brain tissue. The most important achievements in the field of clinical angioneurology include the modern concept of heterogeneity of ischemic stroke, which is based on the idea of the diversity of causes and mechanisms of development of acute focal ischemic brain damage. The amount of reversible and irreversible brain damage largely depends on the state of the hemodynamic, collateral, perfusion and metabolic reserves of the brain. Pathological tortuosity of the main arteries of the head - a hereditarily determined functional inferiority of connective tissue - occurs in at least 10% of the population. Among the main forms of lesions of intracranial arteries, kinks and loop formations, aneurysmal dilatations of arteries, and arteriovenous aneurysms are distinguished. Excessive tortuosity of blood vessels contributes to the formation of blood clots in them. In 71% of patients with arterial occlusion, a tortuous course of vessels was noted. Underdevelopment of the cerebral arteries in the form of hypoplasia or stenosis of the posterior inferior cerebellar artery and/or basilar artery, rarely the inferior anterior cerebellar artery and tortuosity of the vertebral artery are causes of hearing loss and deafness. Weak cerebral artery anastomoses cause cerebral ischemia after cervical discectomy. In Parkinson's and Alzheimer's diseases, neuroimaging and pathological studies have detected cerebrovascular lesions in 20-30% of cases, and vascular disease of the brain may be the basis of dementia. Structural changes in cerebral vessels, a decrease in blood flow velocity and the presence of 30% stenosis in the middle cerebral artery can be prerequisites for the development of stroke in patients with sleep apnea syndrome.
In case of Kimmerle anomaly, it is necessary to take into account the presence of congenital changes in the vertebral arteries. Dysplastic disorders in the area of the craniovertebral junction are 2 times more often noted with pathological tortuosity of the vertebral arteries, to a lesser extent due to hypoplasia of the vertebral arteries. Up to 51.9% of patients with Chiari malformation and syringomyelia have structural features of the cerebral arterial circle. Tortuosity, asymmetry and hypoplasia of the vertebral arteries with signs of impaired blood flow are characteristic of vertebrobasilar insufficiency due to cervical dorsopathy and were noted in 76.6% of cases with central vestibulocochlear syndrome.
The arterial bed of the brain is also affected in such systemic diseases of the body as rheumatoid arthritis, polyarteritis nodosa, Takayasu's disease, Henoch-Schönlein disease. When performing dental procedures in patients with Sturge-Weber disease or Recklinghausen disease, as typical neurofibromatosis, the development of threatening bleeding is possible. Primary damage to the cerebral arteries in the form of vasculitis and endarteritis underlies the development of neurosyphilis and leads to secondary damage to the nervous tissue and the occurrence of infarctions in the brain. Cerebral vasculitis and herpetic cerebral vasculitis can develop against the background of herpetic infection. With ischemic strokes, in 70% of cases in the brain tissue, along with changes caused by acute cerebrovascular accident, focal lesions are observed, similar to changes in meningoencephalitis caused by herpes zoster and herpes simplex.
Changes in the structure of the cerebral arteries must be taken into account when assessing cancer metastases, as well as in 40% of patients with severe traumatic brain injury when identifying traumatic subarachnoid hemorrhages and concomitant angiographic vasospasm.
In the course of carrying out research to study the relationship between variants of human cerebral arteries and cerebrovascular disorders within the framework of acute cerebrovascular accidents and chronic cerebral ischemia, we have developed observations about the relationship between variants of the structure and topography of human cerebral arteries with a number of other nosological units. In this regard, we present the results of our own research on the development of cerebral arteries in humans with various types of epilepsy.
We analyzed the state of the arterial bed of the brain in 748 outpatient and inpatient patients from 22 years to 81 years old, who were examined and treated in the neurological and neurosurgical departments of OKB No. 2 named after. Professor I.N. Alamdarov, Astrakhan in the period 1983-1998, the neurological departments of City Hospital No. 3 and City Hospital No. 4 of Tambov, the neurosurgical department of the Tambov Regional Hospital, the rehabilitation and health complex of the family “B. Lipovitsa", neurological offices and in day hospitals of City Hospital No. 4, Nodal Clinic at the station. Tambov JSC "Russian Railways" and the Central House of Children's Hospital "Tambovmedservice" LLC, as well as in the outpatient clinic "Home Doctor" (Tambov) during 1998-2009.
All patients underwent a comprehensive clinical and instrumental study, including data from a clinical examination by a neurologist, therapist and ophthalmologist, standard laboratory data, electrocardiography, fluorography or plain radiography of the chest organs. According to indications, consultations and examinations were carried out with a neurosurgeon, gynecologist, otolaryngologist, cardiologist, endocrinologist and psychotherapist; a study of the cognitive sphere using a brief mental status assessment scale or a mini-mental status study, transcranial Doppler sonography, electroencephalography. All patients underwent magnetic resonance imaging (MRI) and magnetic resonance angiography (MRA).
According to the data obtained, variants of the structure and topography of the arterial circle of the cerebrum were verified in only 27 cases (61.4% of observations) out of 44 patients with epilepsy or encephalopathies with leading epileptiform syndromes. Among the analyzed group of patients with various types of epilepsy, the following variants of the structure and topography of the arterial circle of the cerebrum were identified: 1) bending of both anterior cerebral arteries in 1 (3.7%) patient; 2) hypoplasia of the left vertebral artery in 2 (7.4%) patients; 3) posterior trifurcation of both internal carotid arteries and anterior trifurcation of the left internal carotid artery, hypoplasia of the basilar artery in 3 (11.1%) patients; 4) anterior trifurcation of the left internal carotid artery, hypoplasia of the basilar artery in 6 (22.2%) patients (Fig. 1); 5) hypoplasia of the right vertebral artery, aplasia of the posterior communicating artery on the right in 4 (14.8%) patients; 6) hypoplasia of the right vertebral artery, hypoplasia of the basilar artery in 5 (18.5%) patients (Fig. 2); 7) anterior trifurcation of the left internal carotid artery, hypoplasia of the posterior communicating artery, hypoplasia of the right posterior cerebral artery in 3 (11.1%) patients; tortuosity of both vertebral arteries in 2 (7.4%) patients; 9) hypoplasia and tortuosity of both vertebral arteries in 1 (3.7%) patient. Below are quite indicative clinical observations from the totality of our own studies.
Among the analyzed group of patients with various types of epilepsy, the following variants of the structure and topography of the arterial circle of the cerebrum were identified: 1) bending of both anterior cerebral arteries in 1 (3.7%) patient; 2) hypoplasia of the left vertebral artery in 2 (7.4%) patients; 3) posterior trifurcation of both internal carotid arteries and anterior trifurcation of the left internal carotid artery, hypoplasia of the basilar artery in 3 (11.1%) patients; 4) anterior trifurcation of the left internal carotid artery, hypoplasia of the basilar artery in 6 (22.2%) patients (Fig. 1); 5) hypoplasia of the right vertebral artery, aplasia of the posterior communicating artery on the right in 4 (14.8%) patients; 6) hypoplasia of the right vertebral artery, hypoplasia of the basilar artery in 5 (18.5%) patients (Fig. 2); 7) anterior trifurcation of the left internal carotid artery, hypoplasia of the posterior communicating artery, hypoplasia of the right posterior cerebral artery in 3 (11.1%) patients; tortuosity of both vertebral arteries in 2 (7.4%) patients; 9) hypoplasia and tortuosity of both vertebral arteries in 1 (3.7%) patient. Below are quite indicative clinical observations from the totality of our own studies.
Example 1. Patient N., 54 years old, is observed in the Nodal clinic at the station. Tambov JSC "Russian Railways" with a diagnosis of encephalopathy as unspecified (epileptic and dyscirculatory) with polymorphic paroxysms (like Todd's palsy) against the background of abnormalities in the structure of the cerebral arteries. Complaints of noise in the head, unsteadiness when walking, attacks of weakness in the right limbs and loss of consciousness. Neurologically: there are no meningeal signs in consciousness, cognitive functions are reduced, emotionally labile, pupils d = s, convergence is weakened, slight weakness of the facial muscles on the right, paresis of the right hand up to 4 points, no sensory disorders, tendon reflexes are increased d > s, staggering in the Romberg position , coordination tests are performed with DetS missing, eyelid tremor. Ophthalmologist: hypertensive angiosclerosis of the retinal vessels of both eyes. Therapist: stage II hypertension, severity of arterial hypertension II, risk category 3. BAC: cholesterol - 4.5 mmol/l, β-lipoproteins - 3.5 g/l, creatinine - 69 µmol/l, urea - 7, 9 mmol/l. Prothrombin - 88%, fibrinogen - 4.25 g/l. ECG: syn, rhythm 80 per minute, normal position of the EOS. MRI and MRA: shown in Figure 1. Outpatient treatment: piracetam, glycine, Cavinton, cinnarizine, aminalon, Enap, Prestarium, indopamine. She underwent a course of treatment in a day hospital: Mexidol, magnesium sulfate, Prestarium, indopamide, mildronate, enalapril. The complaints remain the same. Neurologically - there are no meningeal signs in consciousness, cognitive functions are reduced, cranial nerves without dynamics, paresis of the right hand up to 4.5 points, staggering in the Romberg test, coordination tests with intention on both sides. Consulted at the Tambov psychiatric hospital, where a diagnosis was made: consequences of organic damage to the central nervous system of complex origin with polymorphic paroxysms. EEG: pronounced cerebral changes in biological and bioelectrical activity, practically no α-activity. In all regions the polyri is less than 5 μm. There is no regionality, no activation reaction, no assimilation. Persistent disorganization of the activities of central structures. Interest in the left temporal region, where a focus of slow-wave irritation was identified. Low functional state of the cortex with high sensitivity to hypoxia, with a decrease in the threshold of excitability of the trunk. Single discharges of the posterior trunk sections. Vascular influences are pronounced. Follow-up: while taking Depakine-Chrono, complaints decreased significantly, no attacks were noted.
Example 2. Patient VN., 21 years old, is observed in the Nodal clinic at the station. Tambov JSC Russian Railways with a diagnosis of epilepsy with polymorphic cerebral paroxysms and cognitive impairment. Observed since childhood - complaints of attacks with loss of consciousness. After starting treatment at the age of 14 in the neurological department of the Tambov Regional Children's Hospital, he was registered with a diagnosis of epilepsy, partial seizures with secondary generalization. EEG (Psychiatry and Narcology, 04/17/02): no paroxysmal activity was detected, unstable α-rhythm. Dysfunction of the diencephalic region. Vascular influences with increased autonomic excitability (06/15/04): AMI is moderate, but persistent, with low-amplitude dysrhythmia. Regionality is unclear. The activation reaction is reduced, there is no assimilation. No gross slow-wave or paroxysmal activity was detected. Reduced reactivity of the cortex. High vegetative excitability. Vascular influences 02.22.05: compared to 06.15.04, positive dynamics. General cerebral changes are moderate and of a regulatory nature. Moderate dysfunction of the diencephalic region with increased activity. An unstable α-rhythm appeared, fragmented. The reactivity of the cortex has improved. The sensitivity of the cortex to RFS has increased somewhat, which leads to a decrease in the threshold of excitability of the posterior brainstem structures. There are no paroxysmal tendencies of the cortex. Increased autonomic excitability. MRI and MRA: shown in Figure 2. Neurologically: there are no meningeal signs in consciousness, cognitive functions are slightly reduced, emotionally labile, distal hyperhidrosis, tremor of the eyelids and outstretched fingers, pupils d = s, converges, weakness of facial muscles on the right, in the Romberg position lung staggering, performs coordination tests, paresis, no sensory disorders, tendon reflexes d = s, Babinski's symptom is weakly positive on both sides, Epileptologist: cognitive disorders due to epilepsy. He constantly receives finlepsin (attacks are associated with irregular use of the drug).
Thus, we consider it necessary to draw the following conclusions. 1. Variations in the structure and topography of the arterial circle of the cerebrum can be considered as predictors of epileptiform phenomena in brain structures. 2. For various cerebral paroxysms, it is obviously advisable to study the arterial circle of the cerebrum. 3. Variants of the structure and topography of the arterial circle of the cerebrum in each specific case should be considered from the point of view of minor developmental anomalies.
Ischemic stroke in the posterior cerebral arteries: problems of diagnosis and treatment
I.A. KHASANOV, E.I. BOGDANOV
Republican Clinical Hospital of the Ministry of Health of the Republic of Tatarstan, Kazan
Kazan State Medical University
Khasanov Ildar Akramovich
doctor of the neurological department for patients with acute cerebrovascular accidents
420064, Kazan, st. Orenburgsky Trakt, 138, tel. (843) 237-35-47, e-mail
In the light of modern data, the article examines the problems of diagnosis and treatment of ischemic strokes in the posterior cerebral arteries (PCA), taking into account the characteristics of their etiology, clinical picture and neuroimaging data. The paired posterior cerebral arteries, formed by the bifurcation of the basilar artery and being its terminal branches, serve as the main sources of blood supply to the upper part of the midbrain, the thalami and the posteroinferior parts of the cerebral hemispheres, including the occipital lobes, the mediobasal parts of the temporal lobes and the inferomedial parts of the vertex. Ischemic strokes in the posterior cerebral artery basin account, according to various sources, from 5-10 to 25% of cases of all ischemic strokes. The most common cause of isolated infarctions in the PCA territory is embolic occlusion of the PCA and its branches, which occurs in approximately 82% of cases. In 9% of cases, thrombosis in situ is detected in the PCA; in another 9% of cases, the cause of stroke is vasoconstriction associated with migraine and coagulopathy. A very rare cause of infarction in this region can also be arterial dissection affecting the PCA. The most common and characteristic signs of infarctions in the PCA region are visual disturbances (homonymous hemianopsia), central paresis of the facial nerve, headache, sensory disturbances, aphasic disorders, hemiparesis and nigility.
Key words:
ischemic stroke, cerebral infarction, posterior cerebral artery, neuroimaging, thrombolytic therapy
I.A. KHASANOV, EI BOGDANOV
Kazan State Medical University
Republican Clinical Hospital of the Ministry of Health of the Republic of Tatarstan, Kazan
Ischemic stroke in a system of posterior cerebral arteries: problems of diagnosis and treatment
In the article on the basis of present knowledge are considered the problems of diagnosis and treatment of ischemic strokes in a system of posterior cerebral arteries (PCA) taking into account their causation, clinical presentation and neuroimaging data. Paired posterior cerebral arteries, which are shaped by basilar artery bifurcation and are its terminal branches, are the main sources of blood supply of the upside of midbrain, thalamus and back and bottom parts of cerebral hemispheres, including occipital lobes, mediobasal branches of temporal lobes and lower medial crown branches. Ischemic strokes in a system of posterior cerebral arteries amount to 5-10% or up to 25% of all ischemic strokes. The most common cause of isolated heart attacks
in a system of PCA is the embolic occlusion of PCA and its branches, which occurs in about 82% of cases. In 9% of cases in PCA is revealed thrombosis, in other 9% of cases the cause of stroke are vasoconstriction associated with migraine, and coagulopathy. A very rarely reason for a heart attack in this system can be artery dissection which affects the PCA. The most frequent and characteristic features of heart attacks in a system of PCA are visual impairments (equilateral hemianopsia), central paresis of facial nerve, headache, sensation disorders, aphatic disorders, hemiparesis and neglect.
Key words:
ischemic stroke, cerebrovascular accident, posterior cerebral artery, neuroimaging, thrombolytic therapy.
Ischemic strokes in the posterior cerebral arteries (PCA) account, according to various sources, from 5-10 to 25% of cases of all ischemic strokes [1-4]. They can be the cause of a number of clinical symptoms, which are not always promptly and adequately recognized by the patients themselves, their relatives and doctors, because an acute gross motor deficit, which is usually associated with a stroke, in this case may be unexpressed or completely absent. A delay in timely diagnosis or incorrect diagnosis casts doubt on the possibility of providing the patient with adequate therapy (primarily thrombolysis), which in turn cannot but affect the outcome of the disease [5]. An important role in making a diagnosis is played by the possibility of using neuroimaging, the correct choice of method and competent interpretation of the results [2]. It seems important to present and analyze the features of the clinical picture, neuroimaging and treatment of ischemic strokes in the posterior cerebral arteries in the light of modern data.
The most common cause of isolated infarctions in the PCA territory is embolic occlusion of the PCA and its branches, which occurs in 82% of cases. At the same time, cardiogenic genesis is observed in 41% of cases, while arterio-arterial embolism from the vertebral and basilar arteries is observed in only 32% of cases. In 10% of patients, the source of the embolism cannot be determined. In 9% of cases, thrombosis in situ is detected in the PCA. Vasoconstriction associated with migraine and coagulopathies are the causes of cerebral infarction in 9% of cases [6]. If isolated infarctions in the PCA territory in most cases are of a cardioembolic nature, then involvement of the brainstem and/or cerebellum in combination with an infarction in the PCA territory is most often associated with atherosclerotic lesions of the vessels of the vertebrobasilar system [7, 8]. A very rare cause of infarction in this region can also be arterial dissection affecting the PCA [9]. Regardless of the cause of the infarction, it usually only partially involves the PCA territory [10, 11].
Paired posterior cerebral arteries, formed by the bifurcation of the basilar artery and being its terminal branches, serve as the main sources of blood supply to the upper part of the midbrain, thalamus and posteroinferior parts of the cerebral hemispheres, including the occipital lobes, mediobasal parts of the temporal lobes and inferomedial parts of the vertex [10, 12, 13].
In the early stages of development of the human body, the posterior cerebral artery is a branch of the internal carotid artery (ICA) and is supplied with blood from the carotid system, while the posterior communicating artery (PCA) plays the role of its proximal segment. Subsequently, blood begins to flow into the posterior cerebral arteries from the main artery, and the PCA, being a branch of the internal carotid artery, becomes the most significant anastomosis between the carotid and vertebrobasilar areas. According to various sources, from 17 to 30% of adults have a fetal (embryonic) type of PCA structure, in which the ICA remains the main source of blood supply to the PCA throughout life. The fetal type of PCA structure is in most cases observed unilaterally, with the opposite PCA usually starting from an asymmetrically located, curved basilar artery. In cases where both posterior cerebral arteries are branches of the internal carotid arteries, as a rule, well-developed large posterior communicating arteries are observed, and the superior segment of the basilar artery is shorter than usual (the basilar artery ends with the two superior cerebellar arteries arising from it). In approximately 8% of cases, both PCAs originate from the same ICA [7, 8, 12, 14, 15].
The PCA joins the PCA approximately 10 mm distal to the bifurcation of the basilar artery. Each PCA can be conditionally divided into 3 parts: the precommunication part, or P1 segment according to Fisher, - the section of the PCA proximal to the place where the PCA flows into it, the postcommunication part, or P2 segment, located distal to the place where the PCA flows into the PCA, and the final (cortical) the part that gives off branches to the corresponding areas of the cerebral cortex [12, 16]. The paramedian mesencephalic, posterior thalamoperforating and medial posterior choroidal arteries depart from the precommunicative part, participating primarily in the blood supply to the ventrolateral nuclei of the thalamus and the medial geniculate body. The left and right posterior thalamoperforating arteries may arise from a common trunk called the artery of Percheron; a similar variant of the structure usually occurs in combination with unilateral hypoplasia of the P1 segment and the fetal structure of the PCA. The branches of the postcommunication part are the peduncular perforator, thalamogeniculate and lateral posterior choroidal arteries, supplying the lateral geniculate body, dorsomedial nuclei and thalamic cushion, part of the midbrain and the lateral wall of the lateral ventricle [2, 12, 17]. The main cortical branches of the PCA are the anterior and posterior temporal, parietotemporal and calcarine arteries [10]. The boundaries of the watershed of the middle and posterior cerebral arteries basins fluctuate significantly. Usually the border of the PCA basin is the Sylvian fissure, but sometimes the middle cerebral artery supplies blood to the outer parts of the occipital lobe up to the occipital pole. At the same time, the PCA always supplies blood to areas of the cerebral cortex in the area of the calcarine sulcus, and the optic radiation in some cases receives blood from the middle cerebral artery; accordingly, homonymous hemianopsia does not always imply a heart attack in the PCA territory [12].
With ischemic strokes in the PCA region, depending on the location of the vessel occlusion, as well as on the state of collateral blood supply, the clinical picture may reveal symptoms of damage to the midbrain, thalamus and cerebral hemispheres. In general, up to 2/3 of all infarctions in the PCA territory are cortical, the thalamus is involved only in 20-30% of cases, and the midbrain in less than 10% of cases [7, 18, 19]. Accordingly, the most common variant of ischemic stroke in the PCA basin is an isolated infarction of the cerebral hemispheres, primarily the occipital lobes; combined damage to the thalamus and cerebral hemispheres is less common, in a small percentage of cases - an isolated infarction of the thalamus and, finally, a combination of damage to the midbrain, thalamus and /or hemispheres is the rarest option [2].
Sometimes there is bilateral damage to areas of the brain supplied by blood from the PCA. This occurs primarily in top of the basilar syndrome, which is an embolic occlusion of the distal basilar artery and is characterized by depression of consciousness, visual disturbances, oculomotor and behavioral disorders, often without motor dysfunction [2].
According to a number of authors, the most common and characteristic signs of infarctions in the PCA are visual disturbances (up to 95% of cases), homonymous hemianopia (66.7% of cases), central paresis of the facial nerve (52% of cases), headache, mainly in the occipital region. areas (50 cases), sensory disorders (40% of cases), aphasic disorders (38% of cases), hemiparesis (18% of cases) and niglect (10% of cases). Patients usually have a combination of symptoms [2, 7, 8, 11].
Homonymous hemianopia occurs on the contralateral side with infarctions in the areas of blood supply to the hemispheric branches of the PCA due to damage to the striate cortex, optic radiation or lateral geniculate body. In the absence of occipital pole involvement, macular vision remains intact. The visual field defect may be limited to only one quadrant. Superior quadrant hemianopsia occurs when there is an infarction of the striate cortex below the calcarine sulcus or inferior part of the optic radiation in the temporo-occipital region. Inferoquadrant hemianopsia is a consequence of damage to the striate cortex above the calcarine sulcus or the superior part of the optic radiation in the parieto-occipital region. Occlusion of the calcarine sulcus may also be associated with pain in the ipsilateral eye. Visual disturbances may also be more complex, especially with bilateral occipital lobe lesions, including visual hallucinations, visual and color agnosia, prosopagnosia (agnosia for familiar faces), blindness denial syndrome (Anton syndrome), visual attention deficits, and optomotor agnosia ( Balint's syndrome). Often, visual impairment is accompanied by afferent disorders in the form of paresthesia, disorders of deep, pain and temperature sensitivity. The latter indicate involvement of the thalamus, parietal lobe or brainstem (due to occlusion of the proximal vertebrobasilar region) [2, 8, 10, 20].
Neuropsychological abnormalities associated with PCA infarctions vary significantly and are present in more than 30% of cases. A stroke in the basin of the callosal branches of the left PCA in right-handed people, affecting the occipital lobe and splenium of the corpus callosum, is manifested by alexia without agraphia, sometimes color, object or photographic anomia. Right hemisphere infarctions in the PCA territory often cause contralateral hemiglect. With extensive infarctions involving the medial parts of the left temporal lobe or bilateral mesotemporal infarctions, amnesia develops. Also, with mono- or bilateral mesotemporal infarction, agitated delirium may develop. Extensive infarcts in the territory of the left posterior temporal artery may clinically manifest as anomia and/or sensory aphasia. Thalamic infarctions in the areas of blood supply to the penetrating branches of the PCA can cause aphasia (if the left pillow is involved), akinetic mutism, global amnesia and Dejerine-Roussy syndrome (disorders of all types of sensitivity, severe dysesthesia and/or thalamic pain and vasomotor disturbances in the contralateral half of the body, combined with usually transient hemiparesis, choreoathetosis and/or ballism). Also, infarctions in the PCA region may be associated with dyscalculia, spatial and temporal disorientation. [6, 12, 21, 22].
Bilateral thalamic infarcts are often associated with deep coma. Thus, occlusion of the Percheron artery causes the development of bilateral infarcts in the intralaminar nuclei of the thalamus, which leads to severe impairment of consciousness [2, 12].
Hemiparesis during infarctions in the PCA region occurs in only 1/5 of patients, is often mild and transient and is usually associated with involvement of the cerebral peduncles in the pathological process [23, 24]. Cases of infarctions in the PCA region have been described, when patients exhibited hemiparesis without involvement of the cerebral peduncles. These patients had damage to the distal parts of the PCA, primarily involving the thalamogeniculate, lateral and medial posterior choroidal arteries [23, 25]. It is assumed that hemiparesis during infarctions in the posterior choroidal arteries may be associated with damage to the corticobulbar and corticospinal tracts, even in the absence of visible damage to the internal capsule or midbrain according to neuroimaging data [23]. There are opinions that the development of hemiparesis is associated with compression of the internal capsule by edematous tissue of the thalamus [12].
Infarctions in the PCA territory mimic infarctions in the carotid system in 17.8% of patients [24], especially with combined lesions of the superficial and deep branches of the PCA, which is observed in approximately 38% of cases [7, 19, 26]. Differential diagnosis can be difficult due to the presence of aphasic disorders, nigella, sensory deficits, and usually mild and transient hemiparesis resulting from the involvement of the pyramidal tracts. In addition, memory impairment and other acute neuropsychological disorders can significantly complicate the examination of such patients [2, 18, 19].
Among other conditions that often clinically mimic infarctions in the PCA, we should highlight some infectious diseases (primarily toxoplasmosis), posterior reversible leukoencephalopathy syndrome, neoplastic lesions, both primary and metastatic, and thalamic infarctions caused by deep cerebral vein thrombosis [2 , 27]. Neuroimaging methods often play a decisive role in making a diagnosis.
The main requirements for neuroimaging in the acute period of ischemic stroke are the speed of the study and the information content of the data obtained. The main tasks facing the doctor when using these methods are to exclude a non-ischemic cause of the patient’s symptoms, determine the location and size of ischemic foci and the presence of viable brain tissue, determine the condition of the cerebral vessels, identify cerebral edema and displacement of the midline structures, as well as the presence of hemorrhagic impregnation of ischemic foci. These data should help in quickly determining the patient’s treatment tactics—the possibility of intravenous or intra-arterial thrombolysis, mechanical plaque removal, and brain decompression surgery [28, 29].
Computed tomography (CT) usually does not detect ischemic changes in the brain parenchyma during the first few hours after the onset of stroke, the time most important for initiating therapy, and sometimes even later in the disease. Visualization of the posterior regions of the brain is especially difficult due to artifacts caused by the bones of the skull. However, with strokes in the territory of the PCA, as well as with strokes in the territory of the middle cerebral artery, in some cases, CT may show a hyperintense signal from the PCA itself, which is the earliest sign of a stroke in its territory and is detected in 70% of cases within the first 90 minutes from onset of the disease and in 15% of cases within 12 to 24 hours. This sign appears due to visualization of a calcified embolus or atherothrombosis in situ. On a standard CT scan, the slice plane is parallel to the orbitomeatal line (the line connecting the outer corner of the eye with the external auditory canal and then going to the first cervical vertebra). Based on the course of the SMA, its lumen is usually visualized in one section, which makes it easy to identify hyperdense SMA, especially in the presence of atrophic changes in the brain. The course of the PCA is more complex. Typically, its proximal segment ascends laterally around the cerebral peduncles and, reaching the bypass cistern, goes horizontally inward to the temporal lobe, in close proximity to the tentorium cerebellum. The circular part (P1 and P2 segments) ends in the quadrigeminal cistern, where the cortical part of the PCA begins. Only the P2 segment runs parallel to the cut inside the bypass tank and, accordingly, hyperdensity, if present, can most likely be detected in this area. Subsequently, CT signs of ischemic changes appear as areas of hypointensity in the brain parenchyma [2, 3, 30].
Magnetic resonance imaging (MRI) makes it possible to more accurately determine the presence and nature of ischemic changes in the brain during stroke. Diffusion-weighted imaging (DWI) can detect early ischemic changes, often within an hour of symptom onset, and localize and extend lesions more accurately than CT [2]. The combined use of DWI, ADC and FLAIR modes makes it possible to differentiate acute, subacute and chronic ischemic changes in the brain parenchyma, as well as to distinguish cytotoxic brain edema observed in ischemic stroke from vasogenic edema in the syndrome of posterior reversible leukoencephalopathy and hypertensive encephalopathy [2, 27, 31 , 32].
CT angiography (CTA) plays a significant role in the non-invasive diagnosis of steno-occlusive lesions of large extra- and intracranial arteries. This technique makes it possible to identify the degree of stenosis, plaque morphology, as well as the presence of arterial dissection in both vertebrobasilar and carotid vessels. In addition, the anatomical features of collaterals and circulation options of the PCA are assessed [2, 33, 34]. Additional information about vascular anatomy can be obtained using contrast-enhanced MR angiography, which, in combination with CTA, allows for data that previously could only be obtained using classical angiography. In addition, these methods are important in assessing the effectiveness of thrombolytic therapy in the case of arterial recanalization [2].
Currently, thrombolytic therapy for ischemic stroke can be used for damage to the arteries of both the carotid and vertebrobasilar areas. Nevertheless, all currently existing guidelines for thrombolysis are focused primarily on vascular catastrophe in the carotid region, primarily the middle cerebral artery; this is primarily due to the presence in such patients of obvious neurological deficits in the form of severe paresis and sensory disturbances. A typical functional deficit in a patient with a heart attack in the PCA region in the acute period is not always regarded by the doctor as disabling. The assessment of neurological deficit according to the National Institutes of Health Stroke Scale (NIHSS), which is one of the criteria for selecting patients for thrombolytic therapy, usually is not able to fully reflect the severity of the condition of a patient with a vertebrobasilar infarction [7]. There are no recommendations at all regarding an isolated visual field defect in acute infarction in the PCA territory [2]. Therefore, thrombolytic therapy in patients with infarctions in the PCA is not widely used. However, given that hemiparesis in some cases is a significant clinical component of infarctions in the PCA territory, such patients, in the absence of contraindications, are justifiably treated with systemic and/or intra-arterial thrombolysis [35].
When comparing the efficacy and safety profiles of intravenous thrombolysis administered within the first three hours from the onset of symptoms in patients with carotid infarctions and PCA infarctions, no significant difference in safety and treatment outcome was found [7]. At the same time, according to a number of authors, when conducting intravenous thrombolytic therapy for ischemic lesions in the vertebrobasilar region, and in particular the PCA, it is possible to expand the therapeutic window to 6.5-7 hours and even more compared to 4.5 hours for infarctions in the carotid pool [36, 37].
Intra-arterial thrombolysis for occlusion of the middle cerebral artery is recommended within 6 hours from the onset of symptoms, and for occlusion of the basilar artery - no later than 12 hours [28]. However, to date there are no clear recommendations on the time limits for intra-arterial thrombolysis in patients with PCA lesions [15]. N. Meier et al. (2011) described 9 cases of intra-arterial thrombolysis in patients with PCA occlusion within the first 6 hours from the onset of the disease. 3 months after treatment, functional independence (modified Rankin scale 0-2 points) was detected in 67% of patients, which correlates with similar data for the carotid system [15].
An early diagnosis of ischemic stroke in the PCA allows the doctor to promptly determine the patient’s treatment tactics and, in the absence of contraindications, consider the possibility of thrombolytic therapy, which undoubtedly makes the prognosis for the patient more favorable.
LITERATURE
1. Brandt T., Steinke W., Thie A., Pessin MS, Caplan LR Posterior cerebral artery territory infarcts: clinical features, infarct topography, causes and outcome. Multicenter results and a review of the literature // Cerebrovasc. Dis. - 2000. - Vol. 10. - P. 170–182.
2. Finelli P. Neuroimaging in acute Posterior Cerebral Artery Infarction // The Neurologist. - 2008. - Vol. 14. - P. 170-180.
3. Krings T., Noelchen D., Mull M. et al. The hyperdense posterior cerebral artery sign // Stroke. - 2006. - Vol. 37. - P. 399-403.
4. Hill MD Posterior cerebral artery stroke // e-medicine, 2005.
5. Khasanov I.A. Features of infarctions in the basin of the posterior cerebral arteries // Neurological Bulletin. - 2012. - T. XLIV, issue. 3. - pp. 69-74.
6. Caplan L. Posterior Circulation Ischemia: Then, Now, and Tomorrow: The Thomas Willis Lecture-2000 // Stroke. - 2000. - Vol. 31. - P. 2011-2023.
7. Breuer L., Huttner H. B., Jentsch K. et al. Intravenous Thrombolysis in Posterior Cerebral Artery Infarctions // Cerebrovasc Dis. - 2011. - Vol. 31. - P. 448-454.
8. Caplan L., Bogousslavsky J. Posterior cerebral artery syndromes // Cerebrovascular Disease: Pathology, Diagnosis and Management. - 1998. - P. 1028.
9. Caplan L., Estol C., Massaro A. Dissection of the posterior cerebral arteries // Arch Neurol. - 2005. - Vol. 62. - P. 1138-1143.
10. Brazis P. Topical diagnostics in clinical neurology / P. Brazis, D. Masdew, H. Biller - M.: MEDpress-inform, 2009. - 736 p.
11. Caplan L. Posterior Circulation disease: Clinical Findings, Diagnosis and Management / Boston, MA: Butterworth-Heinemann, 1996. - 533 p.
12. Beer M. Topical diagnosis in neurology according to Peter Duus / M. Beer, M. Frotscher. - M.: Practical Medicine, 2009. - 468 p.
13. Tatu L., Moulin T., Bogousslavsky J. et al. Arterial territories of the human brain // Neurology. - 1998. - Vol. 50 - P. 1699-1708.
14. de Monye C, Dippel DW, Siepman TA et al. Is a fetal origin of the posterior cerebral artery a risk factor for TIA or ischemic stroke? A study with 16-multidetector-row CT angiography // J. Neurol. - 2008. - Vol. 255 - P. 239-245.
15. Meier N., Fischer U., Schroth G. Outcome after thrombolysis for acute isolated posterior cerebral artery occlusion // Cerebrovasc. Dis. - 2011. - Vol. 328. - P. 79-88.
16. Phan T., Fong A., Donnan G. et al. Digital map of posterior cerebral artery infarcts associated with posterior cerebral artery trunk and branch occlusion // Stroke. - 2007. - Vol. 38. - P.1805-1811.
17. Chaves CJ Posterior cerebral artery. Stroke syndromes. 2nd edition / Chaves CJ, Caplan LR Cambridge, New York: Cambridge University Press. — 2001. — 747 p.
18. Cals N., Devuyst G., Afsar N. et al. Pure superficial posterior cerebral artery territory infarction in the Lausanne Stroke Registry // J. Neurol. - 2002. - Vol. 249. - P. 855-861.
19. Kumral E., Bayulkem G., Atac C., Alper Y. Spectrum of superficial posterior cerebral artery territory infarcts // Eur. J. Neurol. - 2004. - Vol. 11. - P. 237-246.
20. Ng YS, Stein J, Salles SS et al. Clinical characteristics and rehabilitation outcomes of patients with posterior cerebral artery stroke // Arch. Phys. Med. Rahabil. - 2005. - Vol. 86. - P. 2138-43.
21. Brandt T., Thie A., Caplan L. et al. Infarkte in Versorgungsgebiet der A. cerebri Posterior // Nervenarzt. - 1995. - Vol. 66. - P. 267-274.
22. Savitz SI, Caplan LR Vertebrobasilar disease // N. Engl. J. Med. - 2005. - Vol. 352. - P. 2618-26.
23. Finelli P. Magnetic Resonance Correlate of Hemiparesis in Posterior Cerebral Artery Infarction // Journal of Stroke and Cerebrovascular Disease. - 2008. - Vol. 17. - P. 378-381.
24. Maulaz AB, Bezerra DC, Bogousslavsky J. Posterior cerebral artery infarction from middle cerebral artery infarction // Arch. Neurol. - 2005. - Vol. 62. - P. 938-941.
25. Neau J.-P., Bogousslavsky J. The syndrome of posterior choroidal artery territory infarction // Ann. Neurol. - 1996. - Vol. 39. - P. 779-788.
26. Lee E., Kang DW, Kwon SU, Kim JS Posterior cerebral artery infarction: diffusion-weighted MRI analysis of 205 patients. Cerebrovasc. Dis. - 2009. - Vol. 28. - P. 298-305.
27. Bogdanov E.I., Khasanov I.A., Mamedov Kh.I. and others. Posterior reversible leukoencephalopathy syndrome in patients with preeclampsia and eclampsia // Neurological Journal. - 2011. - No. 5. - P. 35-40.
28. Adams H., Del Zoppo G., Alberts M. et al. Guidelines for the early management of adults with ischemic stroke // Stroke. - 2007. - Vol. 38. - P. 1655-1711.
29. Wahlgren N., Ahmed N., Davalos A. et al. Thrombolysis with alteplase for acute ischemic stroke & the Safe implementation of thrombolysis in stroke - monitoring study (SITS-MOST): an observational study // Lancet. - 2007. - Vol. 369. - P. 275-282.
30. Berge E., Nakstad PH, Sandset PM Large middle cerebral artery infarctions and the hyperdense middle cerebral artery sign in patients with atrial fibrillation // Acta Radiol. - 2001. - Vol. 42. - P. 261-268.
31. Covarrubias DJ, Leutmer PH, Caumpeau NG Posterior reversible leukoencephalopathy syndrome: prognostic utility of quantitative diffusion-weighted MR image // AJNR Am J. Neuroradiol. - 2002. - Vol. 23, N 6. - P. 1038-1048.
32. Garg R. Posterior leukoencephalopathy syndrome // Postgrad. Med. J. - 2001. - Vol. 77, N 903. - P. 24-28.
33. Choi C., Lee D., Lee J. et al. Detection of intracranial atherosclerotic steno-occlusive disease with 3D time-of-flight magnetic resonance angiography with sensitivity encoding at 3T // AJNR Am J. Neuroradiol. - 2007. - Vol. 28. - P. 439-446.
34. Lev M., Farkas J., Rodrigues V. et al. CT angiography in the rapid triage of patients with hyperacute stroke to intraarterial thrombolysis: accuracy in the detection of large vessel thrombus // J Comput Assist Tomogr. - 2001. - Vol. 25. - P. 520-528.
35. Ntaios G., Spengos K., Vemmou AM et al. Long-term outcome in posterior cerebral artery stroke // European Journal of Neurology. - 2011. - P. 156-162.
36. Forster A., Gass A., Kern R. et al. MR Imaging-Guided Intravenous Thrombolysis in Posterior Cerebral Artery Stroke // AJNR Am J. Neuroradiol. - 2011. - Vol. 32. - P. 419-421.
37. Montavont A., Nighoghossian N., Derex. L et al. Intravenous r-TPA in vertebrobasilar acute infarcts // Neurology. - 2004. - Vol. 62. - P. 1854-1856.