Bisoprolol in the treatment of complex heart rhythm disorders during pregnancy
Heart rhythm disturbances during pregnancy, according to various authors, occur in 20-40% of women (1). The main causes of arrhythmias can be divided into extracardiac, cardiac and arrhythmias, the etiology of which has not been established (idiopathic, primary electrical heart disease).
Table. Blood pressure level and Holter ECG monitoring indicators in pregnant women during treatment with bisoprolol
Extracardiac factors include functional and organic lesions of the central nervous system, dysfunction of the autonomic nervous system with a predominance of the tone of the sympathetic department or a decrease in the tone of the parasympathetic department, endocrine diseases, primarily diseases of the thyroid gland, electrolyte imbalance, mechanical and electrical injuries, hypo- and hyperthermia, excessive physical activity, intoxication with alcohol, nicotine, coffee, medications. Medicines such as sympathomimetics, cardiac glycosides, diuretics, most psychotropic drugs, some antiarrhythmic drugs and antibiotics can cause rhythm and conduction disturbances.
Cardiac factors that can cause cardiac arrhythmias are coronary heart disease, congenital and acquired heart defects, including valve prolapse, arterial hypertension, inflammatory and non-inflammatory myocardial lesions, heart failure, diagnostic procedures and operations on the heart and coronary vessels, some congenital diseases of the cardiovascular system.
Pregnancy, even in practically healthy women, can be a factor provoking the development of heart rhythm disturbances. This is facilitated by gestational changes in the woman’s body relating to hemodynamic, electrophysiological and neurohumoral parameters. In particular, hormones of the sympathetic-adrenal system (SAS) have a proarrhythmogenic effect, the activity of which increases significantly during pregnancy, reflecting the adaptation of the woman’s body to new conditions for the functioning of the “mother-placenta-fetus” system (2, 3). Changes leading to an increase in the activity of the SAS can occur at the level of the central nervous system, which is manifested by a decrease in central inhibitory influences and a decrease in the sensitivity of baroreceptors, an increase in the impulse activity of sympathetic nerves, facilitation of ganglion impulse transmission, increased release of norepinephrine into the synoptic fissure and disruption of its metabolism in this area. cracks. Along with this, the peripheral link of the SAS also changes - the density and/or sensitivity of adrenergic receptors, the interaction of receptor-contractile proteins. The indirect effect of SAS on the level of blood pressure and heart rate occurs through stimulation of the synthesis of renin, vasopressin, the occurrence of insulin resistance, disruption of the functional state of the endothelium and other mechanisms (4,5). All these processes can lead to various heart rhythm disturbances, the treatment of which during pregnancy is a complex and responsible task, because the issue of not only improving the clinical condition and quality of life of a woman, but also the condition of the fetus and newborn is being resolved.
One of the most common heart rhythm disturbances during pregnancy is extrasystole, which in almost half of patients occurs without any organic changes in the cardiovascular system (CVS), gastrointestinal tract, or endocrine system. The main causes of extrasystole during pregnancy are considered physiological changes in hemodynamics, emotional arousal, drinking alcohol, coffee, strong tea, smoking, overeating, and abuse of spicy foods. Of great importance is the electrolyte imbalance towards hypomagnesemia and hypokalemia, the use of drugs - sympathomimetics, caffeine, as well as neurocirculatory dystonia and organic damage to the cardiovascular system, in particular, previous myocarditis, heart defects, cardiomyopathies, etc.
The treatment strategy for cardiac arrhythmias is determined by the basic rule: the prescription of antiarrhythmic drugs should be avoided if the arrhythmia does not pose a threat to the patient’s life. If medication is necessary, treatment approaches are the same as for non-pregnant women. At the same time, the possible effect of the drug on the physiological course of pregnancy, childbirth, and the condition of the fetus and newborn should be taken into account. In Russia there is no classification of drugs according to safety criteria for the fetus, and therefore it is possible to use the American classification of drugs and food products by the Food and Drug Administration (FDA) (6). According to these criteria, there are 5 categories of medicines.
- Controlled studies have shown no risk to the fetus.
- Lack of evidence of risk to the fetus: a risk to the fetus has been found in animals, but not in humans, or there is no risk in the experiment, but there is insufficient research in humans.
- A risk to the fetus cannot be excluded: side effects have been identified in animals, but there is insufficient research in humans. The expected therapeutic effect of the drug may justify its use despite its potential risk to the fetus.
- Strong evidence of risk: There is a proven risk to the fetus in humans, but the expected benefit to the expectant mother may outweigh the potential risk to the fetus.
- Use during pregnancy cannot be justified: a drug that is dangerous to the fetus when the negative effect on the fetus exceeds the potential benefit from this drug in the expectant mother.
The drugs of choice for the treatment of extrasystole during pregnancy are verapamil and beta-blockers.
Beta-blockers (BAB) according to the classification of E. Vaughan-Williams (1971) belong to class II antiarrhythmic drugs. The principal mechanism of the inhibitory effect of beta blockers on adrenoreactive structures is to weaken or eliminate the effects associated with the excitation of β1-adrenergic receptors by catecholamines, which cause increased heart rate, increased automaticity of the atrioventricular node and myocardial excitability, increased impulse conduction speed, increased myocardial contractility, decreased refractory period , activation of lipolysis. Excitation of β2-adrenergic receptors by catecholamines leads to dilation of arterioles, decreased tone of smooth muscles of the bronchi, bladder, uterine tone during pregnancy, tremor of skeletal muscles, inhibition of the release of histamine, leukotrienes in mast cells during allergic reactions of type I, hypokalemia, increased hepatic glycogenolysis (7) . Cardioselective beta blockers contribute to a lesser extent to increasing the tone of peripheral arteries, which is very important during pregnancy, when the peripheral vascular resistance is physiologically reduced, including in the “mother-placenta-fetus” system. The degree of cardioselectivity (effect on β1/β2 receptors) for metoprolol is 1:35, for bisoprolol - 1:75, while for non-cardioselective propranolol the cardioselectivity index was 1.8:1. The later any drug, including beta blockers, is prescribed during pregnancy, the lower the risk of its negative impact on the course of pregnancy and the condition of the fetus. There are reports of a slowdown in intrauterine development of the fetus in women who took propranolol during pregnancy and atenolol in the first trimester of pregnancy, while the use of this drug, like other beta blockers, from the second trimester is considered safe for the fetus and newborn (8). Data from a few randomized studies on the use of beta blockers in the treatment of hypertension during pregnancy have shown their greater clinical efficacy, safety for the fetus and newborn, and the absence of a negative effect on the physiological course of pregnancy and childbirth (9-11).
Cardioselective beta blockers without intrinsic sympathomimetic activity include bisoprolol, which in experimental studies on the reproductive characteristics of animals did not have a teratogenic effect at a dose of 375 and 77 times the MRL, depending on weight and body surface area, respectively, but its fetotoxicity was noted in these supramaximal doses (increased number of late fetal resorptions). The drug has established itself as an active antihypertensive and antiarrhythmic drug in patients with hypertension and arrhythmias of organic origin.
The purpose of the study was to study the clinical effectiveness and safety of bisoprolol in pregnant women with frequent ventricular extrasystole.
Patients and research methods
Under observation in the specialized cardiology department for pregnant women with diseases of the cardiovascular system of City Clinical Hospital No. 67 were 32 patients in the second trimester of pregnancy aged 19-47 years (average age 27.3±3.8 years), of which 30 women were multipregnant and 2 – primigravidas. All patients gave informed consent to participate in the examination and treatment. A comprehensive clinical and laboratory examination included, along with the routine, a blood test for electrolytes - potassium and sodium, thyroid hormones T3, T4, TSH using the radioimmune method, ECG in dynamics, Echo-CG with Dopplerography in continuous and pulsed modes using standard methods on the device “Acuson 128 XP/10” (USA), 24-hour Holter ECG monitoring was performed on the “Medilog Prima” device on days 1-2 of hospital stay and after 3 weeks of treatment with bisoprolol. All pregnant women were observed by an obstetrician. The activity of the sympathetic-adrenal system was assessed by the value of β-adrenoreception of erythrocyte membranes (β-ARM) using the author’s method, based on changes in the osmoresistance of erythrocytes in the presence of a beta-blocker using a set of reagents “ARM-AGAT” (Agat-Med LLC, Moscow) (12) . Bisoprolol (Concor, Nycomed) was used as an antiarrhythmic drug; treatment began with 2.5 mg under the control of blood pressure, heart rate, ECG and subjective state; if the dose was ineffective, it was doubled, and subsequently, if necessary, increased to 10 mg per day. The majority of patients (28 people) received 5 mg of bisoprolol and 4 people. – 10 mg of the drug per day. The course of treatment in hospital averaged 3 weeks. The effectiveness of treatment was assessed by subjective sensations and the results of Holter ECG monitoring.
Statistical processing of the study results was carried out using the “Biostatistics, Version 4.03” software package using standard methods of variation statistics and Student’s test to assess differences in paired measurements of indicators. The difference was considered significant at p
Results and discussion
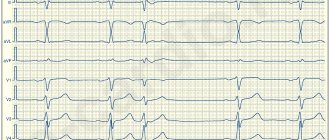
During the initial examination, according to Holter ECG monitoring, frequent ventricular extrasystoles were registered in all examined patients; the number of extrasystoles per day ranged from 8 thousand to 50 thousand, in some (6 people) with couplets (from 13 to 80 per day) and triplets (in 4 people, the number of triplets – from 3 to 150 per day), ventricular tachycardia runs were registered in 5 women (from 1 to 5 per day) with a heart rate from 156 to 229 per minute. These rhythm disturbances corresponded to class III-IV according to the classification of B.Lawn and N.Wolff (1971). Arrhythmia in all patients was manifested by pronounced subjective sensations - a feeling of interruptions and fading of the heart, palpitations, sometimes accompanied by fear, sweating, and weakness. It should be noted that in the majority of subjects (26 people, 81.3%), arrhythmia appeared during pregnancy, in the remaining 6 people. (18.7%) it was present before pregnancy, but with increasing gestational age, subjective tolerability of arrhythmia became worse.
According to the anamnesis and comprehensive clinical and laboratory examination, in half of the patients the cause of arrhythmia was organic or functional changes in the cardiovascular system: corrected congenital heart disease (4 people), dilated cardiomyopathy (2 people), hypertrophic cardiomyopathy without obstruction of the left ventricular outflow tract (1 person .), mitral valve prolapse with mitral regurgitation of II (6 people) and III degree (2 people), post-myocardial cardiosclerosis (1 person). In 16 women, no changes in the cardiovascular system, thyroid gland or biochemical parameters were detected, and these arrhythmias were regarded as idiopathic. As mentioned above, pregnancy causes pronounced hemodynamic changes in a woman’s body due to an increase in body weight due to the growth of the placenta and the increasing weight of the fetus, increased metabolism, the development of physiological hypervolemia, and the formation of uteroplacental blood flow. During gestation, physiological myocardial hypertrophy develops - myocardial mass increases by 10-31% by the end of the third trimester and after childbirth, myocardial mass quickly returns to its original level. In pregnant women, cardiac output increases by 15-50% and stroke volume by 13-29%, blood flow speed increases by 50-83%, heart rate is 15-20 beats per minute higher than pre-pregnancy heart rate, general peripheral vascular resistance decreases by an average of 12-34% (13-15). These hemodynamic factors can act as proarrhythmogenic mechanisms contributing to the development of various cardiac arrhythmias.
The proarrhythmogenic effect is also enhanced by physiological hypersympathicotonia, identified during examination in patients with arrhythmia. Analysis of β-ARM values showed significant fluctuations in this indicator among the examined people - from 25 to 85.5 conventional units. units (on average 39.1±2.8 conventional units). According to the conditions of the method, β-ARM values exceeding 20 arb. units, indicate desensitization of adrenergic receptors under the influence of increased concentrations of endogenous catecholamines in individuals with high SAS activity (11). The high values of β-ARM that we discovered are consistent with the results of other authors who showed that the gestational period is characterized by high functional activity of the SAS (2, 3). It should be noted that in some patients this indicator significantly exceeded the average physiological norms, and in 3 women it reached 70-80.5 conventional units. units Such β-ARM values were recorded in the group of patients with idiopathic extrasystole, which gave us grounds to consider severe hypersympathicotonia as the main proarrhythmogenic factor in this category of patients.
According to the results of treatment, 26 women showed a significant improvement in their clinical condition, which was manifested by a significant decrease in the number of extrasystoles per day according to Holter ECG monitoring (Table 1). It is important to emphasize that paired extrasystoles - doublets and triplets - completely disappeared in 4 patients or decreased by an average of 57% in 3 patients compared to the baseline; no episodes of ventricular tachycardia were recorded. We did not detect a significant decrease in systolic (BPs) and diastolic (BPd) blood pressure.
Treatment with bisoprolol was ineffective in 6 patients (3 of them with organic CVS pathology). One patient had hypertrophic cardiomyopathy, the second had mitral valve prolapse with grade III mitral regurgitation, and the third woman was diagnosed with idiopathic arrhythmia. It should be emphasized that the analysis of the adrenoreactivity indicator revealed a significant increase in all three patients with idiopathic arrhythmia - their β-ARM values exceeded 70 conventional units, while the average for the group β-ARM was 39.1±2. 8 conventional units Such high levels of β-ARM indicate pronounced desensitization of adrenergic receptors, as a result of which there are no “points of application” for beta blockers on the cell membrane. Similar data on adrenergic receptor desensitization under the influence of excessively high concentrations of endogenous catecholamines were obtained in experimental studies in our work, which showed the absence of the hypotensive effect of betaxolol in hypertensive patients with high β-ARM values (16). After inpatient treatment, all 26 patients were recommended to continue taking bisoprolol in an individually selected clinically effective dose, and they continued to be observed by us in the consultative and diagnostic center of City Clinical Hospital No. 67.
An analysis of perinatal outcomes was assessed in 13 of 26 patients observed by us at the consultative and diagnostic center and who gave birth in the specialized maternity hospital of City Clinical Hospital No. 67. All women gave birth independently at 39-40 weeks of pregnancy with a full-term fetus with an Apgar score at the 5th minute 8-9 points. The weight of newborns ranged from 2300 g (in a woman with dilated cardiomyopathy) to 3200-4300 g in all other women. At the same time, researchers note the possibility of symptoms of β-blockade in the form of fetal distress, bradycardia, hypoglycemia and intrauterine growth retardation in children whose mothers took beta blockers (17). In our study, no such or any other complications from the fetus or newborn were noted.
Thus, bisoprolol in pregnant women with complex heart rhythm disorders (class III-IV according to B.Lawn and N.Wolff) is an effective antiarrhythmic drug, does not affect the physiological course of pregnancy and childbirth and does not have a negative effect on the condition of the fetus and newborn.
conclusions
- Bisoprolol is an effective antiarrhythmic drug in the treatment of complex cardiac arrhythmias (class III-IV according to B.Lawn and N.Wolff) in pregnant women with organic diseases of the cardiovascular system and idiopathic arrhythmia.
- In patients with an adrenoreactivity index (β-ARM) of 70 arb. units and more, bisoprolol is ineffective due to pronounced desensitization of adrenergic receptors under conditions of high activity of the sympathetic-adrenal system.
- Treatment of pregnant women with complex heart rhythm disorders with bisoprolol does not affect the physiological course of pregnancy and childbirth and does not have a negative effect on the condition of the fetus and newborn.
- The study of the adrenoreactivity indicator based on the β-ARM value can be a prognostic criterion for the individual sensitivity of patients to β-blockers.
Concor® AM
For amlodipine:
Amlodipine can be safely used for the treatment of arterial hypertension together with thiazide diuretics, alpha-blockers, beta-blockers or ACE inhibitors. In patients with stable angina, amlodipine can be combined with other antianginal agents, for example, long- or short-acting nitrates, beta-blockers.
Unlike other BMCCs, no clinically significant interaction with amlodipine (III generation BMCCs) was detected when used together with non-steroidal anti-inflammatory drugs (NSAIDs)
, including with
indomethacin
.
It is possible to enhance the antianginal and hypotensive effects of BMCC when used together with thiazide and loop diuretics, ACE inhibitors, beta-blockers and nitrates
, as well as enhancing their hypotensive effect when used together with alpha1-blockers and neuroleptics. Although negative inotropic effects have generally not been observed in amlodipine studies, some CBMCs may enhance the negative inotropic effects of antiarrhythmic drugs that cause QT prolongation (eg, amiodarone and quinidine).
Amlodipine can also be safely used concomitantly with antibiotics and oral hypoglycemic agents.
Single dose of 100 mg sildenafil
in patients with essential hypertension does not affect the pharmacokinetic parameters of amlodipine.
Repeated use of amlodipine 10 mg and atorvastatin
at a dose of 80 mg is not accompanied by significant changes in the pharmacokinetics of atorvastatin.
Simvastatin
: Simultaneous repeated use of amlodipine at a dose of 10 mg and simvastatin at a dose of 80 mg leads to an increase in simvastatin exposure by 77%. In such cases, the dose of simvastatin should be limited to 20 mg.
Ethanol (beverages containing alcohol):
amlodipine with single and repeated use at a dose of 10 mg does not affect the pharmacokinetics of ethanol.
Antivirals (ritonavir):
increases plasma concentrations of BMCC, including amlodipine.
Neuroleptics and isoflurane
: increased hypotensive effect of dihydropyridine derivatives.
Calcium preparations
may reduce the effect of BMCC.
When combined with BMCC and lithium preparations
(no data available for amlodipine), possibly increasing the manifestation of their neurotoxicity (nausea, vomiting, diarrhea, ataxia, tremor, tinnitus).
Studies of concomitant use of amlodipine and cyclosporine
in healthy volunteers and all groups of patients, with the exception of patients after kidney transplantation, were not carried out. Various studies of the interaction of amlodipine with cyclosporine in patients after kidney transplantation show that the use of this combination may not lead to any effect, or increase the minimum concentration of cyclosporine to varying degrees, up to 40%. These data should be taken into account and cyclosporine concentrations should be monitored in this group of patients when cyclosporine and amlodipine are co-administered.
Does not affect serum digoxin
and its renal clearance.
Does not significantly affect the action of warfarin
(prothrombin time).
Cimetidine
does not affect the pharmacokinetics of amlodipine.
in vitro studies
amlodipine does not affect the binding of digoxin, phenytoin, warfarin and indomethacin to plasma proteins.
Grapefruit juice:
A simultaneous single dose of 240 mg of grapefruit juice and 10 mg of amlodipine orally is not accompanied by a significant change in the pharmacokinetics of amlodipine. However, it is not recommended to use grapefruit juice and amlodipine at the same time, since genetic polymorphism of the CYP3A4 isoenzyme may increase the bioavailability of amlodipine and, as a result, enhance the hypotensive effect.
Aluminum or magnesium containing antacids:
their single dose does not have a significant effect on the pharmacokinetics of amlodipine.
CYP3A inhibitors4:
with simultaneous use of diltiazem at a dose of 180 mg and amlodipine at a dose of 5 mg in patients from 69 to 87 years of age with arterial hypertension, an increase in the systemic exposure of amlodipine by 57% was observed.
The simultaneous use of amlodipine and erythromycin in healthy volunteers (from 18 to 43) does not lead to significant changes in the exposure of amlodipine (an increase in the area under the curve of “slow” calcium channels (SCCC) such as verapamil and, to a lesser extent, diltiazem, when used simultaneously with bisoprolol may lead to a decrease in myocardial contractility, a pronounced decrease in blood pressure and impaired AV conduction. In particular, intravenous administration of verapamil to patients taking beta-blockers can lead to severe arterial hypotension and AV blockade.
Centrally acting antihypertensives (such as clonidine, methyldopa, moxonidine , rilmenidine)
when used simultaneously with bisoprolol, they can lead to a decrease in heart rate and a decrease in cardiac output, as well as vasodilation due to a decrease in central sympathetic tone. Abrupt withdrawal, especially before discontinuation of beta-blockers, may increase the risk of developing rebound hypertension.
Combinations requiring caution
BMCA dihydropyridine derivatives (for example, nifedipine)
when used simultaneously with bisoprolol, they may increase the risk of developing arterial hypotension. In patients with CHF, the risk of subsequent deterioration in cardiac contractility cannot be excluded.
Class I antiarrhythmics (eg, quinidine, disopyramide, lidocaine, phenytoin, flecainide, propafenone)
when used simultaneously with bisoprolol, they can reduce AV conductivity and myocardial contractility.
Class III antiarrhythmics (eg, amiodarone)
may increase AV conduction disturbances.
Parasympathomimetics
when used simultaneously with bisoprolol, they may increase AV conduction disturbances and increase the risk of developing bradycardia.
The effect of beta-blockers for topical use
(for example, eye drops for the treatment of glaucoma)
may enhance the systemic effects of bisoprolol (lowering blood pressure, lowering heart rate).
Hypoglycemic effect of insulin or oral hypoglycemic agents
may intensify. Beta blockade may mask signs of hypoglycemia, particularly tachycardia. Such interactions are more likely when using non-selective beta-blockers.
Means for general anesthesia
may weaken reflex tachycardia and increase the risk of developing arterial hypotension (see section "Special Instructions").
Cardiac glycosides
when used simultaneously with bisoprolol, they can lead to an increase in AV conduction time and the development of bradycardia.
Nonsteroidal anti-inflammatory drugs
(NSAIDs)
may reduce the antihypertensive effect of bisoprolol.
Concomitant use of bisoprolol with beta-agonists (for example, isoprenaline, dobutamine)
may lead to a decrease in the effect of both drugs.
The combination of bisoprolol with adrenergic agonists
that affect beta and alpha adrenergic receptors (for example, norepinephrine, epinephrine)
may enhance the vasoconstrictor effects of these drugs that occur with the participation of alpha adrenergic receptors, leading to an increase in blood pressure. Such interactions are more likely when using non-selective beta-blockers.
Antihypertensives, as well as other drugs with possible antihypertensive effects (eg tricyclic antidepressants, barbiturates, phenothiazines)
, may enhance the antihypertensive effect of bisoprolol.
Combinations to consider
Mefloquine
when used simultaneously with bisoprolol, it may increase the risk of bradycardia.
MAO inhibitors
(with the exception of MAO-B inhibitors) may enhance the antihypertensive effect of beta-blockers. Concomitant use may also lead to the development of a hypertensive crisis.
Rifampicin
slightly shortens the half-life (T1/2) of bisoprolol. As a rule, no dose adjustment is required.
Ergotamine derivatives
when used simultaneously with bisoprolol, they increase the risk of developing peripheral circulatory disorders.
Concor cor 2.5 mg 30 pcs. film-coated tablets
pharmachologic effect
Beta1-adrenergic blocker selective.
Composition and release form Concor cor 2.5 mg 30 pcs. film-coated tablets
Tablets - 1 tablet:
- active substance: bisoprolol fumarate - 2.5 mg;
- excipients: calcium hydrogen phosphate, anhydrous - 134.0 mg; corn starch, fine powder - 15 mg; colloidal silicon dioxide, anhydrous - 1.5 mg; microcrystalline cellulose - 10.0 mg; crospovidone - 5.5 mg; magnesium stearate - 1.5 mg;
- Film coating: hypromellose 2910/15 - 2.20 mg, macrogol-400 - 0.53 mg, dimethicone-100 - 0.11 mg, titanium dioxide (E 171) - 1.22 mg.
10 tablets per blister made of aluminum foil and PVC; 3 blisters along with instructions for use are placed in a cardboard box.
14 tablets per blister made of aluminum foil and PVC; 1 blister along with instructions for use is placed in a cardboard box.
25 tablets per blister made of aluminum foil and PVC; 2 blisters along with instructions for use are placed in a cardboard box.
30 tablets in a blister made of aluminum foil and PVC; 1 blister along with instructions for use is placed in a cardboard box.
When packaging the drug at the Russian enterprise Nanolek LLC
30 tablets in a blister made of aluminum foil and PVC; 1 or 2 blisters along with instructions for use are placed in a cardboard box.
Description of the dosage form
White, heart-shaped, biconvex, film-coated tablets, scored on both sides.
Directions for use and doses
Concor® Cor tablets should be taken once a day with a small amount of liquid in the morning before, during or after breakfast. The tablets should not be chewed or crushed into powder.
The standard treatment regimen for CHF includes the use of angiotensin-converting enzyme (ACE) inhibitors or angiotensin II receptor antagonists (in case of intolerance to ACE inhibitors), beta-blockers, diuretics and, optionally, cardiac glycosides. Initiation of treatment for CHF with Concor® Cor requires a special titration phase and regular medical supervision.
The precondition for treatment with Concor® Cor is stable chronic heart failure without signs of exacerbation.
Treatment of CHF with Concor® Cor begins in accordance with the following titration scheme. Individual adaptation may be required depending on how well the patient tolerates the prescribed dose, i.e. the dose can only be increased if the previous dose was well tolerated. The recommended starting dose is 1.25 mg once daily. Depending on individual tolerance, the dose should be gradually increased to 2.5 mg, 3.75 mg, 5 mg, 7.5 mg and 10 mg once a day. Each subsequent dose increase should be carried out at least two weeks later.
If increasing the dose of the drug is poorly tolerated by the patient, a dose reduction may be possible.
The maximum recommended dose for CHF is 10 mg of Concor Cor 1 time per day.
During titration, regular monitoring of blood pressure, heart rate and the severity of CHF symptoms is recommended. Worsening of the symptoms of CHF is possible from the first day of using the drug.
If the patient does not tolerate the maximum recommended dose of the drug, a gradual dose reduction is possible.
During the titration phase or after it, temporary worsening of CHF, arterial hypotension or bradycardia may occur. In this case, it is recommended, first of all, to adjust the doses of concomitant therapy drugs. It may also be necessary to temporarily reduce the dose of Concor® Cor or discontinue it.
After stabilization of the patient's condition, the dose should be re-titrated or treatment should be continued.
Duration of treatment
Treatment with Concor® Cor is usually long-term therapy.
Special patient groups
Impaired kidney or liver function:
- Mild or moderate hepatic or renal impairment usually does not require dose adjustment.
- In case of severe renal impairment (creatinine clearance less than 20 ml/min) and in patients with severe liver disease, the maximum daily dose is 10 mg. Increasing the dose in such patients should be carried out with extreme caution.
Elderly patients:
No dose adjustment is required.
Children:
Since there is not enough data on the use of Concor® Cor in children, it is not recommended to prescribe the drug to children under 18 years of age.
To date, there is insufficient data on the use of Concor® Cor in patients with CHF in combination with type 1 diabetes mellitus, severe renal and/or liver dysfunction, restrictive cardiomyopathy, congenital heart defects or heart valve disease with severe hemodynamic disturbances. Also, sufficient data have not yet been obtained regarding patients with CHF with myocardial infarction within the last 3 months.
Pharmacodynamics
A selective beta1-blocker, without its own sympathomimetic activity, does not have a membrane-stabilizing effect. It has only slight affinity for beta2-adrenergic receptors of the smooth muscles of the bronchi and blood vessels, as well as for beta2-adrenergic receptors involved in the regulation of metabolism. Therefore, bisoprolol generally does not affect airway resistance and metabolic processes in which beta2-adrenoreceptors are involved. The selective effect of the drug on beta1-adrenergic receptors persists beyond the therapeutic range.
When used once in patients with coronary heart disease (CHD) without signs of chronic heart failure (CHF), bisoprolol reduces the heart rate (HR), stroke volume of the heart and, as a result, reduces the ejection fraction and myocardial oxygen demand. With long-term therapy, the initially increased total peripheral vascular resistance (TPVR) decreases.
Pharmacokinetics
Suction. Bisoprolol is almost completely (more than 90%) absorbed from the gastrointestinal tract. Its bioavailability due to negligible first-pass metabolism through the liver (at approximately 10%) is approximately 90% after oral administration. Food intake does not affect bioavailability.
Bisoprolol exhibits linear kinetics, with its plasma concentrations being proportional to the dose taken in the range from 5 to 20 mg. The maximum concentration in blood plasma is achieved after 2-3 hours.
Distribution. Bisoprolol is distributed quite widely. The volume of distribution is 3.5 l/kg. The binding to plasma proteins reaches approximately 30%.
Metabolism. Metabolized via the oxidative pathway without subsequent conjugation. All metabolites are polar (water-soluble) and are excreted by the kidneys. The main metabolites found in blood plasma and urine do not exhibit pharmacological activity. Data obtained from in vitro experiments with human liver microsomes indicate that bisoprolol is metabolized primarily by the CYP3A4 isoenzyme (about 95%), with the CYP2D6 isoenzyme playing only a minor role.
Excretion. The clearance of bisoprolol is determined by the balance between excretion by the kidneys unchanged (about 50%) and metabolism in the liver (about 50%) to metabolites, which are then also excreted by the kidneys. The total clearance is 15 l/hour. The half-life is 10-12 hours.
There is no information on the pharmacokinetics of bisoprolol in patients with CHF and concurrent impairment of liver or kidney function.
Indications for use Concor cor 2.5 mg 30 pcs. film-coated tablets
Chronic heart failure.
Contraindications
- Hypersensitivity to bioprolol or to any of the excipients;
- acute heart failure, chronic heart failure in the stage of decompensation, requiring inotropic therapy;
- cardiogenic shock;
- atrioventricular (AV) block II and III degrees, without a pacemaker;
- sick sinus syndrome;
- sinoatrial block;
- severe bradycardia (heart rate less than 60 beats/min);
- severe arterial hypotension (systolic blood pressure less than 100 mm Hg);
- severe forms of bronchial asthma;
- severe peripheral arterial circulation disorders or Raynaud's syndrome;
- pheochromocytoma (without simultaneous use of alpha-blockers);
- metabolic acidosis;
- age under 18 years (insufficient data on effectiveness and safety in this age group).
With caution: carrying out desensitizing therapy, Prinzmetal's angina, hyperthyroidism, type I diabetes mellitus and diabetes mellitus with significant fluctuations in blood glucose concentration, AV block of the first degree, severe renal failure (creatinine clearance less than 20 ml/min), severe liver dysfunction, psoriasis , restrictive cardiomyopathy, congenital heart defects or heart valve disease with severe hemodynamic disturbances, CHF with myocardial infarction within the last 3 months, severe forms of chronic obstructive pulmonary disease, strict diet.
Application of Concor cor 2.5 mg 30 pcs. film-coated tablets during pregnancy and breastfeeding
During pregnancy, Concor® Cor should be recommended for use only if the benefit to the mother outweighs the risk of side effects in the fetus and/or child.
In general, beta blockers reduce blood flow to the placenta and may affect fetal development. Blood flow in the placenta and uterus should be monitored, as well as the growth and development of the unborn child should be monitored, and if adverse events occur in relation to pregnancy and/or the fetus, alternative therapeutic measures should be taken. The newborn should be carefully examined after birth. In the first three days of life, symptoms of bradycardia and hypoglycemia may occur.
There is no data on the excretion of bisoprolol into breast milk. Therefore, taking Concor® Cor is not recommended for women during breastfeeding. If taking the drug during lactation is necessary, breastfeeding should be discontinued.
special instructions
Do not interrupt treatment with Concor® Cor abruptly or change the recommended dose without first consulting your doctor, as this may lead to a temporary deterioration in heart function. Treatment should not be interrupted suddenly, especially in patients with coronary artery disease. If discontinuation of treatment is necessary, the dose should be reduced gradually.
During the initial stages of treatment with Concor® Cor, patients require constant monitoring. The drug should be used with caution in the following cases:
- Severe forms of COPD and non-severe forms of bronchial asthma;
- Diabetes mellitus with significant fluctuations in blood glucose concentration: symptoms of a pronounced decrease in glucose concentration (hypoglycemia), such as tachycardia, palpitations or increased sweating, may be masked;
- Strict diet;
- Carrying out desensitizing therapy;
- AV block of the first degree;
- Prinzmetal's angina;
- Mild to moderate peripheral arterial circulation disorders (increased symptoms may occur at the beginning of therapy);
- Psoriasis (including history).
Respiratory system: for bronchial asthma or COPD, simultaneous use of bronchodilators is indicated. In patients with bronchial asthma, there may be an increase in airway resistance, which will require a higher dose of beta2-agonists. In patients with COPD, bisoprolol prescribed in combination therapy for the treatment of heart failure should be started at the lowest possible dose, and patients should be carefully monitored for the appearance of new symptoms (eg, shortness of breath, exercise intolerance, cough).
Allergic reactions: beta-blockers, including Concor® Cor, may increase sensitivity to allergens and the severity of anaphylactic reactions due to the weakening of adrenergic compensatory regulation under the influence of beta-blockers. Therapy with epinephrine (adrenaline) does not always give the expected therapeutic effect.
General anesthesia: When performing general anesthesia, the risk of beta-adrenergic blockade should be taken into account. If it is necessary to discontinue therapy with Concor® Cor before surgery, this should be done gradually and completed 48 hours before general anesthesia. You should notify your anesthesiologist that you are taking the drug Concor® Cor.
Pheochromocytoma: in patients with an adrenal tumor (pheochromocytoma), Concor®Cor can only be prescribed while using alpha-blockers.
Hyperthyroidism: when treated with Concor® Cor, symptoms of hyperfunction (hyperthyroidism) of the thyroid gland may be masked.
Impact on the ability to drive vehicles and operate machinery
The drug Concor® Cor does not affect the ability to drive vehicles, according to the results of a study in patients with coronary artery disease. However, due to individual reactions, the ability to drive vehicles or operate technically complex mechanisms may be impaired. Particular attention should be paid to this at the beginning of treatment, after changing the dose, and also when consuming alcohol at the same time.
Overdose
Symptoms
The most common symptoms of overdose: AV block, severe bradycardia, marked decrease in blood pressure, bronchospasm, acute heart failure and hypoglycemia.
Sensitivity to a single high dose of bisoprolol varies widely among individual patients and patients with CHF are likely to be highly sensitive.
Treatment
If an overdose occurs, first of all, it is necessary to stop taking the drug and begin supportive symptomatic therapy.
For severe bradycardia: intravenous administration of atropine. If the effect is insufficient, a drug with a positive chronotropic effect can be administered with caution. Sometimes temporary placement of an artificial pacemaker may be necessary.
With a pronounced decrease in blood pressure: intravenous administration of plasma-substituting solutions and vasopressor drugs.
For AV block: Patients should be closely monitored and treated with beta-agonists such as epinephrine. If necessary, install an artificial pacemaker.
In case of exacerbation of CHF: intravenous administration of diuretics, drugs with a positive inotropic effect, as well as vasodilators.
For bronchospasm: prescribing bronchodilators, including beta2-adrenergic agonists and/or aminophylline.
For hypoglycemia: intravenous administration of dextrose (glucose).
Side effects Concor cor 2.5 mg 30 pcs. film-coated tablets
The frequency of the adverse reactions listed below was determined according to the following: very common ≥ 1/10; often ≥ 1/100,
Central nervous system - Often: dizziness, headache; Rarely: loss of consciousness.
General disorders - Often: asthenia, increased fatigue.
Mental disorders - Uncommon: depression, insomnia; Rarely: hallucinations, nightmares.
On the part of the organ of vision - Rarely: reduction of lacrimation (should be taken into account when wearing contact lenses); Very rare: conjunctivitis.
From the organ of hearing - Rarely: hearing impairment.
From the cardiovascular system - Very often: bradycardia; Often: worsening symptoms of CHF; a feeling of coldness or numbness in the extremities, a pronounced decrease in blood pressure; Uncommon: AV conduction disturbance, orthostatic hypotension.
From the respiratory system - Uncommon: bronchospasm in patients with bronchial asthma or a history of airway obstruction; Rarely: allergic rhinitis.
From the digestive tract - Often: nausea, vomiting, diarrhea, constipation; Rarely: hepatitis.
From the musculoskeletal system - Uncommon: muscle weakness, muscle cramps.
From the skin - Rarely: hypersensitivity reactions, such as itching, rash, hyperemia of the skin; Very rare: alopecia. Beta blockers may worsen psoriasis symptoms or cause a psoriasis-like rash.
From the reproductive system - Rarely: potency disorders.
Laboratory indicators - Rarely: increased concentration of triglycerides and activity of “liver” transaminases in the blood (aspartate aminotransferase (AST), alanine aminotransferase (ALT)).
Drug interactions
The effectiveness and tolerability of bisoprolol may be affected by concomitant use of other medications. This interaction can also occur when two drugs are taken within a short period of time. The doctor must be informed about taking other medications, even if taken without a doctor's prescription (i.e., over-the-counter drugs).
Combinations not recommended
Class I antiarrhythmic drugs (for example, quinidine, disopyramide, lidocaine, phenytoin; flecainide, propafenone), when used simultaneously with bisoprolol, can reduce AV conduction and cardiac contractility.
Blockers of “slow” calcium channels (SCBC) such as verapamil and, to a lesser extent, diltiazem, when used simultaneously with bisoprolol, can lead to a decrease in myocardial contractility and impaired AV conduction. In particular, intravenous administration of verapamil to patients taking beta-blockers can lead to severe arterial hypotension and AV block.
Centrally acting antihypertensives (such as clonidine, methyldopa, moxonidine, rilmenidine) can lead to a decrease in heart rate and cardiac output, as well as vasodilation due to a decrease in central sympathetic tone. Abrupt withdrawal, especially before discontinuation of beta-blockers, may increase the risk of developing “rebound” arterial hypertension.
Combinations requiring special caution
BMCC dihydropyridine derivatives (for example, nifedipine, felodipine, amlodipine) when used simultaneously with bisoprolol may increase the risk of arterial hypotension. In patients with CHF, the risk of subsequent deterioration in cardiac contractility cannot be excluded.
Class III antiarrhythmic drugs (eg, amiodarone) may worsen AV conduction disturbances.
The effect of beta-blockers for topical use (for example, eye drops for the treatment of glaucoma) may enhance the systemic effects of bisoprolol (lowering blood pressure, lowering heart rate).
Parasympathomimetics, when used simultaneously with bisoprolol, may enhance AV conduction disturbances and increase the risk of developing bradycardia.
The hypoglycemic effect of insulin or oral hypoglycemic agents may be enhanced. Signs of hypoglycemia - in particular tachycardia - may be masked or suppressed. Such interactions are more likely when using non-selective beta-blockers.
General anesthesia agents may increase the risk of cardiodepressive effects, leading to hypotension.
Cardiac glycosides, when used simultaneously with bisoprolol, can lead to an increase in impulse conduction time, and thus to the development of bradycardia.
Nonsteroidal anti-inflammatory drugs (NSAIDs) may reduce the hypotensive effect of bisoprolol.
The simultaneous use of Concor® Cor with beta-agonists (for example, isoprenaline, dobutamine) may lead to a decrease in the effect of both drugs. The combination of bisoprolol with adrenergic agonists that affect beta and alpha adrenergic receptors (for example, norepinephrine, epinephrine) may enhance the vasoconstrictor effects of these drugs that occur with the participation of alpha adrenergic receptors, leading to an increase in blood pressure. Such interactions are more likely when using non-selective beta-blockers.
Antihypertensive drugs, as well as other drugs with a possible antihypertensive effect (for example, tricyclic antidepressants, barbiturates, phenothiazines) may enhance the hypotensive effect of bisoprolol.
Mefloquine, when used simultaneously with bisoprolol, may increase the risk of bradycardia.
MAO inhibitors (except MAO B inhibitors) may enhance the hypotensive effect of beta-blockers. Concomitant use may also lead to the development of a hypertensive crisis.
How to reduce the risk of venous thromboembolism when taking COCs
In the previous article, I told you about the main risks of taking oral contraceptives - deep venous thrombosis and pulmonary embolism. This is a life-threatening condition, which, of course, is better not to reach. Today we will discuss the main risk factors in order and see how they should be taken into account.
It's not possible - it's not possible
First, do not allow women who have contraindications to take COCs in accordance with the National Medical Criteria for Contraceptive Methods and WHO criteria (5th revision, 2015).1
Consider combinations of various factors that require caution
The benefit/risk ratio is carefully assessed in patients with several conditions/diseases for which COCs are not contraindicated, but require caution (2nd class of acceptability).
For example, age over 40 years and obesity with a BMI = 30 kg/m2 are not contraindications, but the combination of these two factors is quite explosive. The large-scale cohort study European Active Surveillance Study on Oral Contraceptives (EURAS-OC), conducted in 2000–2005, demonstrated the association of the risk of venous thromboembolism with age and weight: the older the patient and the higher her weight, the higher the likelihood of developing venous thrombosis.
Don't do pointless tests
In most cases, before starting COC use, the likelihood of venous thromboembolism cannot be predicted using tests. No coagulogram or D-dimer will allow you to assess how the blood coagulation system will behave.
It has now become very fashionable to conduct genetic studies to identify mutations in the genes of the hemostatic system before prescribing COCs to everyone. This, of course, is very interesting and exciting, but it is only necessary for those who have had early strokes, heart attacks or thromboembolism among their close relatives. In fact, mutations are quite rare and are not a 100% indicator that trouble will definitely happen.
By the way, about “genetic risk”. In recent years, a number of publications have appeared in the scientific literature indicating that carriers of blood groups II, III and IV have 2 times higher chances of getting venous thrombosis than carriers of the first group. This phenomenon was even called “non-zero blood type.” So, a non-zero blood group is a stronger risk factor for thrombosis than heterozygous polymorphisms of the II and V factor genes. But we do not deny the opportunity to take COCs to all carriers of blood group II (A) (and they are the majority in our population)!
Consider the “travel syndrome” (or “economy class syndrome”)
The connection between venous thrombosis and travel was first noted on flights across the Atlantic Ocean. It is now known that the risk lies not on the plane, but in a long-term forced sedentary position during flights or transfers lasting more than 6 hours2. In such situations, it is advisable for all COC users to use compression knitwear of the 1st class, choose an aisle seat, walk frequently, and do exercises for the calf muscles.
If you have surgery
Surgery itself is a serious thrombogenic factor. That is why COCs should be discontinued at least 2 weeks before planned operations, resuming use no earlier than 3 weeks after complete restoration of physical activity. Minor surgeries lasting up to 30 minutes do not increase the risk of thrombotic complications, but it is best to discuss this issue with your doctor3. Surgical treatment of varicose veins is not associated with a high risk of venous thromboembolism.
Avoid unnecessary “breaks”
It is absolutely no secret that the risk of thromboembolic complications is highest in the first months of taking COCs - during the period of adaptation to the drug. After just 3 months, the risk decreases significantly and by the end of the first year it becomes even lower than the population average.
I have always been terribly perplexed by the mysterious love for “rest breaks” while taking COCs. As soon as the body adapted to the drug, the risk of dangerous events decreased significantly. It would seem, take your pills and enjoy life. But no! It is imperative to take a break for 1-3 months, either after 6 months, or after 9, or after a year, forcing the body to go through the most difficult period of adaptation again and raise the risk of thrombosis to new heights4.
How the risk of venous thrombosis is reduced5
Incidence of venous thromboembolism vs duration of COC use. EURAS study.
Note The increased risk is mainly concentrated in the first year (more precisely, in the first 3 months) after starting use.
A “rest” from contraceptives for a woman in need of contraception is fraught not only with the onset of an unwanted pregnancy, but also with quite serious complications. In the end, it is absolutely unclear why the body, which is in a monotonous dormant state while regularly taking COCs, is so “tired” of it.
For the vast majority of healthy women of reproductive age, the well-established benefits of COCs outweigh the very low absolute risk of venous thromboembolism, but awareness of this and the use of appropriate precautions is certainly necessary.
1. World Health Organization. Medical Eligibility Criteria for Contraceptive Use, 4th edition. Geneva: WHO, 2009. WHO. Medical eligibility criteria for contraceptive use. Fifth edition. Geneva: WHO August 2015. https://www.who.int/reproductivehealth/publications/family_planning/MEC-5/en/ 2. Russian clinical guidelines for the diagnosis, treatment and prevention of venous thromboembolic complications (VTEC) // Phlebology. - 2015. - No. 4, issue 2. - P. 1–52. 3. Contraception and thrombosis. Ways to reduce thrombotic risk in women using COCs: information bulletin / G. B. Dicke, A. V. Solovyova; edited by V. E. Radzinsky. - M.: Editorial office of the magazine StatusPraesens, 2016. - 16 p. 4. Dinger JC, et al. The risk of venous thromboembolism in OC users: time patterns after initiation of treatment // Pharmacoepidemiol Drug Saf 2010; 19 (S1); S214-5. 5. Figure adapted from Suissa, et al. //Hum Reprod 2000; 15:817–21.
Oksana Bogdashevskaya
Photo istockphoto.com
Concor®
Termination of therapy and “withdrawal syndrome”
You should not abruptly interrupt treatment with bisoprolol or change the recommended dose without first consulting your doctor, as this may lead to a temporary deterioration in heart function.
Treatment should not be interrupted suddenly, especially in patients with coronary artery disease (worsening attacks of angina pectoris, the development of myocardial infarction and the occurrence of ventricular arrhythmias in patients with coronary artery disease have been noted when taking beta-blockers suddenly). If discontinuation of treatment is necessary, the dose of bisoprolol should be reduced gradually. In case of significant worsening of angina or development of acute coronary syndrome, bisoprolol should be temporarily resumed.
Diseases for which the drug should be used with caution
Bisoprolol should be used with caution in the following cases:
- severe forms of COPD and non-severe forms of bronchial asthma;
- diabetes mellitus with significant fluctuations in blood glucose concentrations: bisoprolol may mask symptoms of hypoglycemia (a marked decrease in blood glucose concentrations), such as tachycardia, palpitations or increased sweating;
- strict diet;
— carrying out desensitizing therapy;
— atrioventricular block of the first degree;
- vasospastic angina (Prinzmetal's angina); cases of coronary spasm have been observed. Despite its high beta1 selectivity, angina attacks cannot be completely excluded when taking bisoprolol in patients with Prinzmetal's angina. You should take the drug with extreme caution;
- mild to moderate peripheral arterial circulation disorders (increased symptoms may occur at the beginning of therapy);
- psoriasis (including history).
Diseases of the cardiovascular system
Beta blockers should not be used in decompensated chronic heart failure until the patient's condition has stabilized.
At the initial stages of using bisoprolol, patients need constant monitoring.
Beta blockers may cause bradycardia. If the resting heart rate decreases to less than 50-55 beats/min, the dose should be reduced or discontinued taking bisoprolol.
Like other beta blockers, bisoprolol may cause a prolongation of the PQ interval on the ECG. Bisoprolol should be used with caution in patients with first degree atrioventricular block.
Non-selective beta-blockers may increase the frequency and duration of anginal attacks in patients with vasospastic angina (Prinzmetal's angina) due to alpha-receptor-mediated coronary artery vasoconstriction.
Cardioselective beta-blockers (including bisoprolol) should be used with caution in vasospastic angina.
To date, there is insufficient data regarding the use of bisoprolol in patients with CHF in combination with type 1 diabetes mellitus, severe renal and/or liver dysfunction, restrictive cardiomyopathy, congenital heart defects or heart valve disease with severe hemodynamic disturbances. Also, sufficient data have not yet been obtained regarding patients with CHF with myocardial infarction within the last 3 months.
Respiratory system
Despite the fact that selective beta-blockers have a lesser effect on the function of the respiratory system than non-selective beta-blockers, patients with chronic obstructive pulmonary disease COPD and mild forms of bronchial asthma should be prescribed bisoprolol with extreme caution and only if possible the benefits of its use outweigh the potential risks.
For bronchial asthma or COPD, simultaneous use of bronchodilators is indicated. In patients with bronchial asthma, there may be an increase in airway resistance, which requires a higher dose of beta2-agonists.
In patients with COPD, bisoprolol prescribed in combination therapy for the treatment of heart failure should be started at the lowest possible dose, and patients should be carefully monitored for the appearance of new symptoms (eg, shortness of breath, exercise intolerance, cough).
Major surgery and general anesthesia
If surgical interventions are necessary, the anesthesiologist should be warned that the patient is taking beta-blockers (risk of drug interactions with the development of severe bradyarrhythmias, reduction of reflex tachycardia and arterial hypotension). It is recommended not to stop taking bisoprolol in the perioperative period unless clearly necessary (since beta-adrenergic receptor blockade reduces the risk of arrhythmias and myocardial ischemia during induction of anesthesia and tracheal intubation).
If it is necessary to interrupt treatment with bisoprolol before surgery, the drug should be discontinued at least 48 hours before surgery.
Pheochromocytoma
In patients with pheochromocytoma, bisoprolol can only be prescribed while using alpha-blockers.
Thyrotoxicosis
With hyperthyroidism, beta-blockers (including bisoprolol) can mask tachycardia and reduce the severity of symptoms of thyrotoxicosis. Abrupt withdrawal of the drug can cause exacerbation of symptoms of the disease and the development of thyrotoxic crisis.
Hypersensitivity reactions
Beta blockers, including bisoprolol, may increase sensitivity to allergens and the severity of anaphylactic/hypersensitivity reactions due to decreased adrenergic compensatory regulation by beta blockers. The use of usual therapeutic doses of epinephrine (adrenaline) while taking beta-blockers does not always lead to achieving the desired clinical effect.
Caution should be exercised when prescribing bisoprolol to patients with a history of severe hypersensitivity reactions or undergoing desensitization.
Psoriasis
When deciding on the use of bisoprolol in patients with psoriasis, the expected benefits of the drug should be carefully weighed against the possible risk of exacerbation of psoriasis.
Contact lenses
Patients who use contact lenses should take into account that the use of beta-blockers may reduce the production of tear fluid.
Afobazole
An original anxiolytic (anti-anxiety) drug.
Afobazole is a non-benzodiazepine anxiolytic and has a new mechanism of action: through the sigma receptor system, it is able to activate the natural anti-anxiety defense of nerve cells (“endogenous anxiolysis system”).
Afobazole has a special clinical profile that is different from all other anti-anxiety drugs:
- the effect develops from the first week of administration and persists after completion of treatment;
- does not cause daytime sleepiness, dependence and addiction, as well as withdrawal syndrome;
- has not only an anti-anxiety, but also an activating effect;
- suitable for the treatment of sleep disorders associated with anxiety, as well as premenstrual syndrome and withdrawal syndrome when quitting smoking;
- compatible with most other somatotropic drugs, does not interact with ethanol.
Question answer
Is it possible to take Afobazol together with herbal sedatives and glycine?
Afobazole does not interact with herbal sedatives and glycine, so it can be taken together. However, when using combination therapy, you should consult your doctor.
What is the maximum duration of Afobazolm therapy?
Afobazole does not accumulate in the body, does not cause addiction or dependence, which makes it possible to safely carry out long courses of therapy. The duration of a course of drug use is usually 2–4 weeks; if necessary, the duration of treatment can be extended up to 3 months. As a rule, the duration of the course depends on the initial condition, its changes during therapy, concomitant diseases, and the presence of external stress factors. Upon completion of the course of therapy, it is necessary to consult with your doctor to decide on further treatment tactics.
How often can Afobazole courses be repeated? How much break should I take between courses?
Afobazole can be taken without interruption for 3 months. After stopping treatment, the effect of the drug lasts for 1-2 weeks. The duration of breaks between courses of therapy depends on your condition and is determined in each individual case by the attending physician based on the results of an assessment of your condition.
Is Afobazol compatible with alcohol?
The simultaneous use of Afobazole and alcohol is not contraindicated. However, it must be remembered that alcohol can have an adverse effect on the central nervous system, including in the presence of anxiety disorders. In addition, drinking high doses of alcohol is harmful to the body and can increase the symptoms of various diseases.
Hello, is it possible to use Afobazole together with birth control pills?
No interaction between Afobazole and oral contraceptives has been identified; joint use is not contraindicated.
How long before you can plan a pregnancy after stopping the drug?
Afobazole is quickly eliminated from the body. At the same time, after discontinuation of any drug, it is necessary to ensure that the symptoms of the condition for which the drug was prescribed have completely resolved and there is no need to continue treatment with this or any other drug. Therefore, we recommend planning pregnancy no earlier than 2 weeks after discontinuation of the drug.
Tell me, is it possible to take Afobazole during pregnancy? Thank you in advance
During pregnancy, the use of many medications is contraindicated, incl. Afobazole. Despite the fact that, according to experimental studies, Afobazole does not have a negative effect on fetal development in animals, the effects of Afobazole in pregnant women have not been sufficiently studied.
Hello, is it possible to take Afobazole while driving a car?
Afobazole does not have a sedative effect, and also does not cause a deterioration in concentration and speed of psychomotor reactions, therefore it can be taken by people driving vehicles whose activities require increased attention and quick response.
Can the drug be used in children?
According to the approved instructions, Afobazol is not used in children under 18 years of age, because No special studies have been conducted in this category of people.
Hello, is it possible to take Afobazole while taking antidepressants?
The simultaneous use of Afobazole and antidepressants is not contraindicated.
Can the drug help the first time you use it?
The drug Afobazole reduces the severity of anxiety disorders of various origins. The therapeutic effect does not develop immediately, but gradually. And although the first improvement can be felt quite quickly, a noticeable effect occurs on the 5-7th days of treatment. The optimal duration of therapy is 2-4 weeks; if necessary, taking Afobazole can be continued for up to 3 months.
Is it possible to take Afobazol during lactation?
Due to the lack of clinical data on the use of Afobazole during breastfeeding, the drug should not be taken during lactation. If it is necessary to take it, you should consider stopping breastfeeding.
What side effects can Afobazole cause?
Side effects of Afobazole include: allergic reactions; in rare cases, headaches have been described, which usually go away on their own and do not require discontinuation of the drug.
In what cases is the use of Afobazole contraindicated?
Taking Afobazole is contraindicated in case of individual intolerance to the drug, during pregnancy, during breastfeeding, as well as in children under 18 years of age.