Treatment of patients with ventricular arrhythmias
T
The term “ventricular ectopic arrhythmias” refers to single and group ventricular complexes emanating from foci located below the bifurcation of the His bundle.
Classification
Ventricular ectopic arrhythmias are divided into 3 main categories: extrasystole, or premature ventricular contractions; ventricular tachycardia; ventricular flutter and fibrillation.
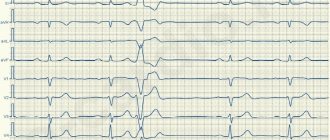
Ventricular extrasystoles are single or paired complexes emanating from the ventricles. For many years, cardiologists have widely used the classification proposed by B. Lown and M. Wolf [1], dividing ventricular extrasystoles into five gradations: rare isolated; frequent (more than 1 per minute); polymorphic; group; early. Later, a modified classification was proposed and has now become widespread [2], which involves the division of ventricular arrhythmias according to their shape and frequency of extrasystoles
(Table 1).
A special classification of ventricular tachycardia has also been proposed [2].
, volley, unstable and persistent forms
are distinguished based on the morphology
of the ventricular complexes on the ECG, tachycardia is distinguished as monomorphic, polymorphic, “pirouette”, bidirectional and emanating from the outflow tract of the right ventricle.
Clinical significance
It is known that an increase in gradations of ventricular arrhythmias in patients with organic heart disease and a decrease in its pumping function is associated with an increased risk of sudden arrhythmic death [1, 3, 4]. In this regard, T. Bigger [4] proposed to distinguish three categories of ventricular arrhythmias:
benign, potentially malignant, malignant.
He considers extrasystoles to be benign, regardless of their gradation, occurring in persons without organic heart damage. Such extrasystoles do not affect the life prognosis. The author classifies as potentially malignant ventricular extrasystoles that occur with a frequency of more than 10 per minute in patients with organic heart disease with decreased contractility of the left ventricle. He classifies as malignant paroxysms of persistent ventricular tachycardia, episodes of ventricular fibrillation, eliminated with the help of resuscitation measures in patients with organic heart diseases, especially with a decrease in left ventricular ejection function of less than 40%. The combination of high-grade ventricular arrhythmias and decreased left ventricular contractility significantly increases the risk of sudden arrhythmic death
.
In patients with coronary heart disease, in whom sudden death occurs more often, there is another important risk factor: acute myocardial ischemia, which, with the two above, forms the so-called risk triangle for sudden arrhythmic death [5]. The relationship between the above risk factors is presented schematically in Figure 1. It is known that an increase in gradations of ventricular arrhythmias in patients with organic heart damage and a decrease in its pumping function is associated with an increase in the risk of sudden arrhythmic death [1, 3, 4].
In this regard, T. Bigger [4] proposed to distinguish between benign, potentially malignant, and malignant. He considers extrasystoles to be benign, regardless of their gradation, occurring in persons without organic heart damage. Such extrasystoles do not affect the life prognosis. The author classifies as potentially malignant ventricular extrasystoles that occur with a frequency of more than 10 per minute in patients with organic heart disease with decreased contractility of the left ventricle. He classifies as malignant paroxysms of persistent ventricular tachycardia, episodes of ventricular fibrillation, eliminated with the help of resuscitation measures in patients with organic heart diseases, especially with a decrease in left ventricular ejection function of less than 40%. . In patients with coronary heart disease, in whom sudden death occurs more often, there is another important risk factor: acute myocardial ischemia, which, with the two above, forms the so-called risk triangle for sudden arrhythmic death [5]. The relationship between the above risk factors is presented schematically in Figure 1. Fig. 1. Risk factors for sudden arrhythmic death in patients with coronary artery disease
Ventricular arrhythmias of high grades are the most important sign of electrical instability of the myocardium. Other known markers of this condition may be disturbances in the autonomic regulation of heart rate with a predominance of sympathetic activity, which is manifested by a decrease in sinus rate variability and baroreceptor sensitivity [6, 7], an increase in the duration and dispersion of the QT interval [8], as well as the appearance of late ventricular potentials recorded using high-resolution ECG [9]. Changes in QT interval dispersion and detection of late ventricular potentials have little specificity in identifying individuals at increased risk of sudden arrhythmic death.
The main risk factors for sudden arrhythmic death, their clinical manifestations and methods of identification in patients with coronary artery disease are presented in Table 2. The issue of risk factors, methods for their identification and approaches to the prevention of sudden death was described in detail in one of our previous works [10].
Treatment
Speaking about the tactics of treating cardiac arrhythmias in general and ventricular arrhythmias in particular, it is necessary first of all to note that they do not always require special therapy. Malignant and potentially malignant arrhythmias require treatment primarily. There are three indications for antiarrhythmic therapy:
unfavorable prognostic value of arrhythmia;
negative impact of arrhythmia on hemodynamics; poor subjective tolerance of arrhythmia. Speaking about the tactics of treating cardiac arrhythmias in general and ventricular arrhythmias in particular, it is necessary first of all to note that they do not always require special therapy.
Malignant and potentially malignant arrhythmias require treatment primarily. There is an unfavorable prognostic value of arrhythmia; negative impact of arrhythmia on hemodynamics; poor subjective tolerance of arrhythmia. A very common erroneous medical tactic is the unjustifiably aggressive prescription of antiarrhythmic drugs for the treatment of benign arrhythmias. This tactic can cause great harm to the patient, because Most antiarrhythmic drugs have serious side effects; long-term use of some of them can negatively affect life prognosis. A significant negative consequence of this tactic is that the patient, who previously did not pay much attention to cardiac sensations, seeing the attention that the doctor pays to the treatment of arrhythmia, begins to listen to the work of his heart, constantly checks his pulse and considers himself seriously ill, which is far from always true. Another mistake is a complacent attitude towards arrhythmias that actually require adequate therapy. Therefore, the first question that a doctor must answer when examining a patient with arrhythmia is the question of the need and advisability of antiarrhythmic therapy and the purpose of the latter. The goals of such therapy are not only and not so much the complete elimination of arrhythmia (which is often unrealistic), but rather the improvement of the prognosis and quality of life of the patient.
In an effort to eliminate arrhythmia, it is necessary first of all to keep in mind its etiology, i.e. underlying disease. In some cases, etiotropic therapy is enough to ensure that arrhythmia ceases to be a therapeutic problem. In addition, when examining a patient, it is necessary to identify pathogenetic factors and conditions that contribute to the onset and cessation of arrhythmia, for example, psycho-emotional reactions, physical stress, parasympathetic influences, electrolyte imbalance, intoxication, arrhythmogenic effects of medications, etc. Elimination of these factors can play an important role in treatment of arrhythmias. Thus, in persons without organic heart disease who have subjectively poorly tolerated ventricular extrasystole, psychotropic drugs can be successfully used; if there is a tendency towards bradycardia, anticholinergic drugs can be used; and for electrolyte imbalances, potassium and magnesium drugs can be used.
The question of the use of antiarrhythmic drugs for the treatment of ventricular arrhythmias in patients with organic heart pathology is very complex and has many contradictory aspects. The most widespread study of the results of the use of antiarrhythmics, based on the classification of EM Vanghan Williams as modified by JC Harrison [11], although it is known that many antiarrhythmics have the properties of not one, but several classes, and their assignment to one of the groups according to their predominant action is very arbitrary.
The results of the use of antiarrhythmic drugs in patients who have had myocardial infarction have been most studied. CAST–I studies
and
CAST-II
showed that suppression of ventricular extrasystoles using class I drugs flecainide, encainide and moricizine led to a significant increase in the risk of sudden and all-cause mortality [12].
A negative impact on the life prognosis of post-infarction patients was noted when using drugs of class I A and I B [13]. It should be noted that in all studies that showed a negative effect of class I antiarrhythmics on life prognosis, these drugs were given for a long time, continuously and in large doses. However, in a retrospective analysis of the results of the CAST studies, suppression of ventricular extrasystoles with low doses of antiarrhythmics may help reduce the risk of sudden death [14]. Class I antiarrhythmic drugs can be safely used to treat ventricular arrhythmias in patients with non-coronary myocardial diseases that are not accompanied by a marked decrease in myocardial contractility. etacizin
,
allapinin
[15] and
propafenone
are common in our country .
Adrenergic b-receptor blockers can play a significant role in the treatment of ventricular arrhythmias
.
Numerous randomized clinical studies have shown that beta-blockers that do not have their own sympathomimetic activity, including cardioselective ones, can significantly reduce the mortality of post-infarction patients, in particular, the incidence of sudden death [13, 17]. It was noted that the reduction in mortality does not always coincide with the elimination of ventricular arrhythmias and may be due not so much to antiarrhythmic effects as to antiadrenergic, antianginal and other effects. There is evidence that combined therapy with β-blockers and class I C antiarrhythmics in the CAST studies led to a decrease in mortality in post-infarction patients [18]. The randomized clinical trial CIBIS-II showed that the use of the cardioselective beta-blocker bisoprolol
in patients with heart failure of various nature helps reduce mortality and suppress malignant ventricular arrhythmias [19]. It is known that drugs of this class can be successfully used for the treatment of ventricular arrhythmias in non-coronary heart diseases, in particular, those accompanied by left ventricular hypertrophy, myocardial dystrophies of various origins, as well as idiopathic arrhythmias.
As for class IV drugs (calcium channel blockers), they are usually ineffective for the treatment of ventricular arrhythmias, with the exception of rare cases of ventricular tachycardia, sensitive to verapamil [20].
Class III drugs (potassium channel blockers that slow down repolarization) play an important role in the treatment of ventricular arrhythmias. There are so-called “pure” potassium channel blockers, for example, dofetilide, ibutilide, azimilide, etc., which have not yet entered clinical practice in Russia, and drugs that, in addition to the ability to slow down repolarization, have properties of other classes. The latter, in particular, includes sotalol
(meaning the popular d/l - sotalol) and
amiodarone
.
Sotalol has the properties of a non-selective b-blocker. There are a number of reports on the results of randomized studies showing the high effectiveness of this drug in the treatment and prevention of ventricular arrhythmias, and the antiarrhythmic effectiveness and patient survival were higher than when using class I drugs [21, 22]. However, studies using sotalol have shown a fairly high incidence of side effects, in particular, an arrhythmogenic effect, the likelihood of which increases with increasing dosage of the drug [23].
Role of amiodarone
Amiodarone is currently the most widely used antiarrhythmic drug. Like other class III drugs, it is able to block potassium channels and prolong the action potential, slowing repolarization. In addition, the drug inactivates fast sodium channels, like class I antiarrhythmics, and can also block slow calcium channels, like class IV drugs. Amiodarone also has a non-competitive inhibitory effect on a- and b-adrenergic receptors, i.e. has a sympatholytic effect. Thus, amiodarone has the properties of all four classes of antiarrhythmic drugs. A significant role in the antiarrhythmic effect of amiodarone is played by its ability to inhibit the synthesis of thyroxine in the thyroid gland and the conversion of the latter to triiodothyronine.
Amiodarone reduces myocardial oxygen demand and causes dilation of the coronary arteries, which causes the antianginal effect of the drug. Unlike most antiarrhythmics, the negative inotropic effect of amiodarone is insignificant. Amiodarone is slowly absorbed from the gastrointestinal tract and binds to plasma proteins. The maximum plasma concentration of amiodarone is achieved several hours after administration. The drug is deposited in adipose tissue, skeletal muscles, liver and other organs. The half-life of amiodarone can range from several weeks to three months. This property causes the slow onset and long duration of action of amiodarone. The antiarrhythmic effect of the drug, when administered intravenously, reaches its maximum only after a few hours, and when taken orally, it begins after 2–3 days and reaches a maximum after several weeks. After amiodarone is stopped, its effects may continue for several weeks [24].
Despite the fact that the high antiarrhythmic effectiveness of amiodarone has been known since the early 70s, this drug became widespread only in the 90s, when the results of randomized studies became known, showing the possibility of a negative effect of class I antiarrhythmics on life prognosis, and also studies that revealed the ability of amiodarone to reduce mortality in post-infarction patients. Such valuable properties of the drug as a low frequency of arrhythmogenic effects and the absence of a pronounced negative inotropic effect, along with high efficiency, have brought amiodarone to first place in terms of frequency of prescription among all antiarrhythmics.
The high effectiveness of amiodarone in the relief and prevention of relapses of atrial fibrillation and flutter is well known. According to the summary data of seven randomized studies, the reversing effectiveness of intravenous amiodarone for paroxysms of atrial fibrillation and flutter is on average 66.5%, not inferior to propafenone, procainamide, disopyramide and other drugs [25].
Amiodarone is even more effective in preventing relapses of atrial fibrillation. According to a Canadian comparative study completed in 2000 [26], amiodarone ranks first in terms of preventive effectiveness for this arrhythmia, surpassing propafenone and sotalol.
Amiodarone is highly effective for the relief and prevention of nodal reentrant tachycardia and tachycardia attacks in patients with ventricular preexcitation syndrome.
The drug is one of the most effective drugs for the treatment of ventricular extrasystole of various origins, however, given the fairly high frequency of undesirable effects with long-term use (see below), it is advisable to prescribe this drug mainly for malignant forms of arrhythmia that are resistant to antiarrhythmic drugs of other classes [27 ].
Amiodarone, when administered intravenously, can stop paroxysms of ventricular tachycardia [28]. Considering the slow action of the drug, it is better to use it not as an initial remedy, but in cases resistant to therapy with lidocaine and other class I drugs.
The use of amiodarone is of greatest importance for the prevention of life-threatening ventricular arrhythmias.
.
As mentioned above, this concept refers to ventricular arrhythmias of high grades (Table 1) in patients with reduced left ventricular ejection fraction, as well as primary ventricular fibrillation. In 1993, the results of the CASCADE
[29], which assessed the survival of patients resuscitated after primary ventricular fibrillation during therapy with amiodarone or class I antiarrhythmics (quinidine, procainamide, flecainide).
The results of this study showed that the preventive effectiveness of amiodarone was significantly and significantly higher compared to class I drugs. This was manifested by higher survival of patients and a lower frequency of recurrence of arrhythmias. In the 90s, a large number of randomized studies of the effect of amiodarone on the survival of post-infarction patients with an increased risk of sudden death were conducted, in particular, BASIS
[30],
CAMIAT
[31],
EMIAT
[32], etc. Their results showed a significant reduction in the incidence of sudden death. death during treatment with amiodarone.
Several randomized studies have been devoted to studying the use of amiodarone in patients with circulatory failure of various etiologies. In particular, the results of the Argentine study GESICA
[33] showed that in the group of patients treated with amiodarone, both total and sudden mortality were significantly lower than in the control group.
In another randomized trial, CHF-STAT
[34], overall mortality was lower, but not significantly, in the amiodarone-treated group, and a decrease in the number of ventricular extrasystoles was noted in this group.
Pooled results from two meta-analyses of a large number of randomized trials on the use of amiodarone in patients at recurrent risk of sudden death were recently published. In one of them, L. Sim et al. [35], based on generalized data from 15 studies, noted a significant reduction in sudden and total mortality during treatment with amiodarone in groups of patients with myocardial infarction with left ventricular dysfunction and survivors of circulatory arrest. ATMA
study [36], based on the results of 13 randomized trials of patients with myocardial infarction and heart failure patients with potentially malignant ventricular arrhythmias, noted that the use of amiodarone leads to a significant reduction in the risk of both arrhythmic death and death from all causes. Data from this meta-analysis also supported the finding that the addition of beta-blockers to amiodarone leads to an additional reduction in the risk of death. In all of these studies, amiodarone was used in low to moderate doses. When trying to use large (more than 1000 mg per day) doses of the drug in the form of long-term administration in one of the studies [37], an increase in mortality was noted compared to the control group, and therefore the study was stopped.
Summarizing the results of these studies using amiodarone, we can state that this drug is currently the most effective and safe among antiarrhythmics in patients with a high risk of sudden death.
, implantation of cardioverter-defibrillators has increasingly become part of clinical practice.
(ICD) to reduce the risk of death in patients with malignant ventricular arrhythmias. An analysis of the currently published results of studies comparing the effectiveness of this method with treatment with amiodarone [38–41], although it generally indicates a greater effectiveness of ICD, gives conflicting results and does not allow us to unambiguously recommend this method as a means of choice for the prevention of sudden death . Combination therapy with ICD and antiarrhythmic drugs, in particular amiodarone, is also possible, but this may pose complex problems of device-drug interactions [42], which have not yet been sufficiently studied.
Side effects of amiodarone
Treatment options with amiodarone are limited to a certain extent by its undesirable effects, which are more often observed when using high doses of the drug [24, 27, 36]. The most serious of these is the development of torsades de pointes (TdP), which can transform into ventricular fibrillation (acquired long QT interval syndrome). This arrhythmia can occur with rapid saturation with high doses of amiodarone or when the latter is combined with other antiarrhythmics that slow down ventricular repolarization. Other arrhythmogenic effects are also possible, in particular, an increase in the frequency of previously existing or the emergence of new ventricular arrhythmias. The arrhythmogenic effect of amiodarone occurs much less frequently than with the use of other antiarrhythmics, in particular, classes III and I. When using amiodarone in low doses, the frequency of arrhythmogenic effects, according to various authors, ranges from 1 to 5%. Often the reason for stopping or interrupting amiodarone is the development of severe sinus bradycardia, sinoatrial or atrioventricular block. According to ATMA
[36], bradycardia causes drug withdrawal in 2.4% of cases.
Of the extracardiac side effects, the most serious is interstitial pneumonitis or pulmonary fibrosis, which develops with long-term use of high doses of the drug with a frequency of up to 1%.
One of the most common side effects of amiodarone is thyroid dysfunction. With long-term use of the drug, approximately 5% of patients develop hypo- or hyperthyroidism. Amiodarone should not be prescribed to patients with thyroid dysfunction.
With long-term continuous use of amiodarone, 1–6% develop skin pigmentation, most often of a gray-greenish tint, accompanied by a change in photosensitivity and increased sensitivity to sunlight. Long-term treatment with this drug may cause pigment deposits in the corneal epithelium, which occasionally leads to minor visual disturbances.
Other side effects of amiodarone include nausea, headache, insomnia, and, less commonly, other neurological disorders that develop in a small percentage of patients. In some patients, the activity of liver enzymes increases, although clinical manifestations of liver dysfunction are rare. Amiodarone increases plasma digitalis. In this regard, when prescribing amiodarone and digitalis drugs simultaneously, the doses of the latter should be reduced.
It should be emphasized once again that almost all of the listed side effects are usually observed with long-term use of high doses of amiodarone. With more careful administration of the drug in a maintenance dose of up to 200 mg per day intermittently, the risk of developing undesirable effects is significantly reduced.
Treatment tactics for arrhythmias
The foregoing implies the need to be careful when prescribing amiodarone, as well as other antiarrhythmics. I would like to warn doctors against insufficiently justified prescribing of these drugs, which, unfortunately, often occurs. An empirical approach is possible to select therapy for patients with benign arrhythmias. For malignant arrhythmias, to select and evaluate the effectiveness of treatment, it is necessary to use the ECG Holter monitoring method, as well as electrophysiological examination of the heart
.
I would like to warn against the unreasonable prescription of high doses of antiarrhythmics, because this significantly increases the risk of side effects. It is necessary to select the minimum effective dose. In particular, the use of high doses of amiodarone can be considered justified only when stopping dangerous arrhythmias. For paroxysms of ventricular tachycardia, this drug is administered in a stream of 150 mg, and then dropwise over several hours in a total daily dose of about 1000 mg. When taken orally, the stopping daily dose of amiodarone can be up to 2000 mg. For persistent arrhythmias, treatment with amiodarone is started with a dose of 400–800 mg per day for several days, and then switched to maintenance doses. The most common regimen is that to maintain the effect, the drug is prescribed at 200 mg per day, 5 days a week.
Having achieved the desired result in benign arrhythmias, taking antiarrhythmics can be stopped, resuming it as needed, avoiding long-term continuous use of drugs. For potentially malignant and malignant arrhythmias, we also consider the use of intermittent treatment tactics with antiarrhythmics, especially amiodarone, possible in many cases when a stable remission is achieved, the absence of relapses of arrhythmias for several weeks, along with an improvement in hemodynamic parameters, a decrease in the frequency of anginal attacks, etc. This can be greatly facilitated by treatment of the underlying disease, in particular, the use of ACE inhibitors (enalapril)
, antiplatelet agents, statins, lifestyle improvement, etc.
In conclusion, it should be noted that correctly selected, careful therapy with antiarrhythmic drugs can today be considered the leading method of treating patients with ventricular arrhythmias.
The list of references can be found on the website https://www.rmj.ru
Amiodarone –
Amiocordin (trade name)
(KRKA)
Enalapril –
Renipril (trade name)
(ICN Pharmaceuticals)
References:
1. Lown B., Wolf M. Approaches to sudden death from coronary heart disease. Circulation, 1971,44, 130–142
2. Myerburg RJ, Huikuri HV, Castellanos A. Origins, classification and significance of ventricular arrhythmias. In: Spooner PM, Rosen MR ed. Foundations of Cardiac Arrythmias. New York, Basel, Marcel Dekker Inc., 2001. 547–569
3. Vismara LA, Amsterdam BA, Mason DT Relation of ventricular arrhythmias in the late hospital phase of acute myocardial infarction to sudden death after hospital discharge. Am. J. Med., 1975, 5, 6–12
4. Bigger JT Identification of patients at high risk for sudden cardiac death. Am. J. Cardiol., 1984, 54, 3D–8D
5. Goldstein S., Bayes-de-Luna A., Guindo-Soldevila J. Sudden cardiac death. Armonk, Futura, 1994, 13–26
6. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Heart rate variability. Standards of measurement physiological interpretation and clinical use. Eur. Heart J., 1996, 17, 354–379
7. La Rovere MT, Bigger JT, Marcus FI, et al. Baroreflex sensitivity and heart–rate variability in prediction of total cardiac mortality after myocardial infarction. ATRAMI Investigators. Lancet, 1998, 351, 478–484
8. Zabel M., Klingenheben T., Franz MR et al. Assessment of QT dispersion for prediction of mortality of arrhythmic events after myocardial infarction: results of a prospective long–term follow–up study, Circulation, 1998, 97, 2543–2550
9. Ivanov G.G., Okhlopkova T.G., Popov V.V. and others. Late ventricular potentials in patients with different forms of coronary heart disease: implications for long-term prognosis and evaluation of therapy. Cardiology, 1998, No. 11, 28–33
10. Doshchitsin V.L. Sudden arrhythmic death and threatening arrhythmias. Russian Journal of Cardiology, 1999, No. 1, 46–51
11. Harrison DC Antiarrhythmic drug classification: new science and practical applications. Am. J. Cardiol., 1985, 56, 185–187
12. Epstein AE, Bigger JT, Wyse DS et al. Events in the Cardiac Arrhythmia Suppression Trial (CAST): Mortality in the entire population enrolled. J. Am. Coll. Cardiol., 1991, 18, 14–19
13. Teo KK, Yusuf S., Furberg CD Antiarrhythmic Drug Therapy in Acute Myocardial Infarction. JAMA, 1993, 270, 1589–1595
14. Goldstein S., Brooks MM, Ledingham R. et al. Association between ease of suppression of ventricular arrhythmia and survival. Circulation, 1995, 91, 79–83
15. Pevzner A.V., Bakalov S.A., Malakhov V.I. and others. Results of the use of allapinin, etacizin and bonnecor in the treatment of patients with paroxysmal ventricular tachyarrhythmias using intracardiac electrophysiological study as a control method. Cardiology, 1996, No. 6, 52–57
16. Dobrotvorskaya T.E., Koroleva O.N., Gordina O.V. and others. Clinical experience with the use of propafenone (ritmonorma) for rhythm disturbances in patients with coronary heart disease. Clinical Medicine, 1996, No. 3, 51–53
17. Krumholz HM, Radford MJ, Wang Y. et al. National use and effectiveness of beta-blockers for the treatment of elderly patients after acute myocardial infarction. JAMA, 1998, 280, 623–629
18. Kennedy HL, Brooks MM, Barker AH et al for the CAST investigators. Beta-blocker therapy in the cardiac arrhythmia suppression trial. Am. J Cardiol 1994, 74, 674–680
19. The Cardiac Insufficiency Bisoprolol Study II (CIBIS – II): A Randomized trial. CIBIS–II Investigators and Committees. Lancet, 1999, 353, 9–13
20. Strasberg B., Kusniec J., Lewin RF et al. An unusual ventricular tachycardia responsive to verapamil. Am. Heart J., 1986, 111, 190–192
21. Mason JW for the Electrophysiologic Study vs Electrocardiographic Monitoring (ESVEM) investigators – A comparison of electrophysiologic testing with holter monitoring to predict antiarrhythmic drug efficacy for ventricular tachyarrhythmias. N.Engl. J. Med., 1993, 329, 445–451
22. Reiffel JA, Hahn E, Hartz V et al. Sotalol for ventricular tachyarrhythmias: beta–blocking and class III contributions and relative efficacy vs class I drugs after prior drug failure. Am. J. Cardiol., 1997, 79, 1008–1053
23. MacNeil DJ, Davies RO, Deichman D. Clinical safety profile of sofalol in the treatment of arrhythmia. Am. J. Cardiol., 1993, 72, 44A–50A
24. Marcus FI Clinical pharmacology of amiodarone. Ann. NY Acad. Sci., 1984, 427, 112–125
25. Preobrazhensky D.V., Sidorenko B.A., Lebedeva O.V. and others. Amiodarone (Cordarone): place in modern antiarrhythmic therapy. Clinical pharmacology and therapy. 1999, 8 (4), 71–77
26. Roy D., Talajic M., Dorian P. et al. Amiodarone to prevent recurrence of atrial fibrillation. N.Engl. J. Med., 2000, 342, 913–920
27. Kushakovsky M.S. – Cardiac arrhythmias (2nd edition). St. Petersburg, “Foliant”, 1999, 99–104
28. Scheinman M., Levine JH, Cannon D. et al. Dose-ranging study of intravenous amiodarone in patients with life-threatening ventricular tachyarrhythmias. Circulation, 1995, 92, 3264–3272
29. The CASCADE Investigators. Randomized antiarrhythmic drug therapy in survivors of cardiac arrest. Am. J. Cardiol., 1993, 72, 280–287
30. Burkart E., Pfisterer M., Kiowski W. et al. Effect of antiarrhythmic therapy on mortality in survivors of myocardial infarction with asymptomatic complex ventricular arrhythmia. Basel Antiarrhythmic Study on Infarct Survival (BASIS), J. Am. Coll. Cardiol., 1990, 16, 1711–1718
31. Cairns JA, Connolly SJ, Roberts R. et al – Randomized trial of outcome after myocardial infarction in patients with frequent or repetitive ventricular premature depolarization: CAMIAT. Lancet, 1997, 349, 675–682
32. Julian DG, Camm AJ, Frangin G. et al. Randomized trial of effect of amiodarone on mortality in patients with left–ventricular dysfunction after recent myocardial infarction: EMIAT. Lancet, 1997, 349, 667–674
33. Doval H., Nul D., Grancelli H. et al for de Estudio de la Sobrevida en la Insuficiencia Cardiaca en Argentina (GESICA) – Randomized trial of low-dose amiodarone in severe congestive heart failure. Lancet, 1994, 344, 493–498
34. Singh B., Fletcher R. et al – Amiodarone in patients with congestive heart failure and asymptotic ventricular arrhythmia. N.Engl. J. Med., 1995, 333, 77–82
35. Sim J., McDonald K., Laveri P. Quantitative overview of randomized trials of amiodarone to prevent sudden cardiac death. Circulation, 1997, 96, 2823–2829
36. Amiodarone Trials Meta–Analysis (ATMA) investigators effect of prophylactic amiodarone on mortality after myocardial infarction and congestive heart failure, meta–analysis of individual data from 6500 patients in randomized trials. Lancet, 1997, 350, 1417–1427
37. Elizari MV, Martinez JM, Belziti C. et al on behalf of the GEMICA Study Investigators. Morbidity and following early administration of amiodarone in acute myocardial infarction. European Heart J., 2000, 21, 198–205
38. Siebels J., Kuck K. and The CASH Investigators. Implantable cardioverter defibrillator compared with antiarrhythmic drug therapy in cardiac arrest survivors (The Cardiac Arrest Study Humburg). Am. Heart J., 1994, 127, 1139–1144
39. Conolly SJ, Gent M, Roberts RS et al. Canadian Implantable Defibrillator Study (CIDS). Am. J. Cardiol., 1993, 72, 103F–108F
40. The Antiarrhythmics vs Implantable Defibrillators (AVID) investigators. A comparison of antiarrhythmic drug therapy with implantable defibrillators in patients resuscitated from near–fatal ventricular arrhythmia. N.Engl. J. Med., 1997, 337, 1576–1583
41. Moss AJ, Hall WJ, Cannom DC et al. Improved survival with an implanted defibrillator in patients with coronary disease of high risk for ventricular arrhythmia. Multicentral Autonomic Defibrillator Implantation Trial (MADIT) Investigators. N.Engl. J. Med., 1996, 335, 1933–1940
42. Golitsyn S.P. The boundaries of benefit and risk in the treatment of ventricular arrhythmias. International Journal of Medical Practice, 2000, 10, 56–64