From this article you will learn: how doctors identify pacemaker migration, why it occurs, and whether it is dangerous. How can it bother a person, is it possible to get rid of it.
Author of the article: Victoria Stoyanova, category 2 doctor, head of the laboratory at the diagnostic and treatment center (2015–2016).
Article publication date: 06/19/2017
Article updated date: 02/08/2020
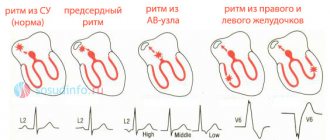
Pacemaker migration is the movement of the source of the impulse that makes the heart beat from one place to another. Normally, the impulse should always be formed in the sinus node. It is located at the top of the right atrium. When the impulse begins to be generated by different parts of the atria, we are talking about migration of the pacemaker.
Normal impulse formation in the sinus node
Pacemaker migration is dangerous because it can be a sign of cardiovascular disease. It also increases the tendency to atrial arrhythmias, such as atrial flutter.
If your ECG reveals this feature, consult a cardiologist or arrhythmologist.
If the migration of the pacemaker was caused by a disease, then it can be eliminated only after the underlying disease has been completely cured. If it is not accompanied by any pathologies, it may disappear on its own with age.
Causes and mechanism of occurrence
Pacemaker migration is not always a disease; sometimes in a healthy part of the population this syndrome is considered a variant of the norm, as a physiological feature of the body.
However, such cases are observed infrequently, since pathology is more often found in organic or functional lesions of the heart or other organs. The main reasons for migration of the supraventricular pacemaker are presented in the table.
| Non-cardiac diseases |
|
| Heart diseases |
|
In the presence of what complaints can pathology be suspected?
There are no complaints that reliably belong to this condition. Most often, pacemaker migration is diagnosed randomly during an electrocardiographic examination. It should be noted that in practice, there are supraventricular (supraventricular) and ventricular forms (very rare) pathologies.
Patients may feel:
- fatigue;
- decreased performance;
- a feeling of interruptions in the work of the heart;
- attacks of rapid heartbeat.
Quite rarely, symptoms of cardialgia, shortness of breath with difficulty breathing, dizziness, and episodes of syncope (short-term loss of consciousness) occur.
How dangerous is the disease?
In most cases, cardiac arrhythmia in a child has a favorable prognosis and is benign. However, for any form of the disease, it is extremely important to monitor the baby’s health, regularly conduct preventive examinations and, according to the doctor’s decision, undergo treatment.
In the absence of competent and timely treatment, the pathology can cause serious complications in the future. The long course of the disease has a detrimental effect on the functioning of the heart and leads to dangerous consequences.
Arrhythmia can cause heart failure and cardiomyopathy, which carry the risk of early disability and even death. Increases the risk of developing arterial hypotension and myocardial ischemia. A prolonged attack of tachycardia can provoke cardiogenic shock or acute heart failure with pulmonary edema.
In addition, the long-term chronic course of the pathology significantly worsens the child’s well-being. The quality of life is gradually decreasing, and constant fatigue and apathy only increase over the years Source: Dubovaya A.V. Modern approaches to assessing the quality of life of children with arrhythmias Russian Bulletin of Perinatology and Pediatrics, 2016; 61:5, pp. 75-81.
Features in children
The most common cause of rhythm source migration in children is vegetative-vascular dystonia. The pathology is characterized by a change in the tone of the vascular wall. The sensitivity of the structures and tissues of the arterial endothelium contributes to functional disorders of areas of the conduction system of the heart, causing migration of the pacemaker to the atrioventricular node. For more information about what VSD is and how to deal with it, see the video at the link below. Brief, accessible and about the main thing from the experts of our portal.
In addition, changes in the source of the rhythm can be observed with endocarditis or in the presence of a pathological athlete's heart. In adolescence, the picture of the disease becomes more pronounced. There are constant complaints of headaches and chest pains, slow development, and sleep disturbances.
Extracardiac factors
In addition to strictly cardiac issues, there are other reasons of an objective nature that do not depend on the patient’s behavior and habits:
- Vegetovascular dystonia. Or VSD. Contrary to the claims of many experts, it is not considered a diagnosis. This is a symptomatic complex.
It is typical for patients with past or current pathologies of the central nervous system and brain appendages. It can also be caused by hormonal imbalance.
In this case, the deviation is associated with activation of the vagus nerve. Requires urgent identification of the origin, then relief of symptoms and prevention of recurrent attacks is indicated.
- Viral and infectious-inflammatory pathologies. From simple colds and acute respiratory viral infections to tuberculosis and other dangerous conditions. As treatment progresses, the results vary. In the absence of gross defects, complete restoration is likely.
- Endocrine disorders. Hyperthyroidism, excessive synthesis of hormones of the adrenal cortex, excess of specific substances of the pituitary gland, androgens, angiotensin, aldosterone, renin.
Treatment consists of normalizing the background. As you achieve this goal, your health improves. But if defects in the development of the heart occur, as an option, cardiomyopathy, full compensation cannot be expected.
How is the diagnosis confirmed?
To make a diagnosis, an integrated approach is used to determine the cause and additional signs of developing heart failure.
Algorithm for assessing the patient’s condition:
- medical history taking into account concomitant pathologies;
- physical examination: inspection, palpation and percussion of the chest;
- auscultation (listening) of heart sounds and additional noises;
- laboratory methods: general and biochemical blood tests (cholesterol, lipid profile, glucose, indicators of the functional state of the kidneys).
The most reliable method of diagnosis is electrocardiography, which records the electrical activity of various areas.
Pathology on the cardiogram is characterized by:
- violation of the shape and duration of the P wave;
- decrease in heart rate;
- changing PQ intervals;
- shortening or slowing down the RR interval.
Additionally, other instrumental diagnostic methods are used:
- Holter monitoring - makes it possible to register the location of the emergence of a new source of impulses, the duration and time of appearance of the pacemaker migration. The study also determines the reasons that led to this condition.
- EchoCG (echocardiography) - detects pathological changes in the structure of the heart (damage, inflammation or abnormalities of the walls and valves).
Migration of the pacemaker on the ECG in a child is characterized by severe bradycardia, which causes characteristic clinical signs.
Heart rhythm disturbances in undifferentiated connective tissue dysplasia
Disturbances in cardiac rhythm and conduction in connective tissue dysplasia (CTD) are recorded quite often (
). Thus, according to Peretolchina T. F. (2000), electrocardiographic (ECG) examination reveals certain abnormalities in 2/3 of patients with undifferentiated connective tissue dysplasia (UCTD), and Holter monitoring (HM) - in 95%. According to our observations, in a group of patients with varying degrees of severity of UCTD, rhythm and conduction disturbances are recorded in 64.4% of cases.
The clinical significance of arrhythmias is different: in some patients, rhythm disturbances, causing cardiac discomfort, can affect the quality of life without significantly affecting the prognosis; in others - often without a clear connection with subjective tolerability - they can be potentially life-threatening; In some cases, rhythm and conduction disturbances can manifest as sudden cardiac death. The results of currently available studies suggest that in patients with UCTD, the pathogenesis of cardiac arrhythmias is multifactorial in nature, which determines their wide spectrum, different prognostic significance and different treatment and preventive approaches.
Disturbances in the function of automatism of the sinus node. As a reflection of excessive sympathetic influences, sinus tachycardia is most often recorded.
In patients with CM, daily fluctuations in heart rate are recorded from 54 to 120–130 beats/min, while on the resting ECG the heart rate (HR) does not exceed normal values in half the cases. The number of episodes of sinus tachycardia can vary from 10 to 416 during the day, the maximum volume in most cases occurs during the period of active physical activity [10]. Sinus arrhythmia is often detected (RRmax–RRmin > 0.15 s): in 10.0–43.2% of cases [4]. In 15–20% of patients with UCTD, mainly at night during sleep, short-term episodes of sinus bradycardia can be recorded - from 1 to 427 s, according to O. V. Tikhonova (2006), the duration of the maximum episode is 2 minutes 11 seconds. The author notes an increase in the number and duration of episodes in patients with a severe form of UCTD [10]. Sinus bradycardia and arrhythmia are more often observed in patients with initial vagotonia.
Pacemaker migration. This variant of arrhythmias is detected in 5.0–34.0% of patients [5, 8, 10]. The frequency of detection of this rhythm disorder almost doubles when conducting an electrophysiological study [11]. More often there is migration of the pacemaker between the sinus and atrioventricular nodes: the heart is excited under the influence of impulses emanating sequentially from the sinus node, atria, atrioventricular junction and again the sinus node [6]. During pacemaker migration, the leading role of the sinus node is temporarily suppressed by ectopic pacemakers. Despite the fact that the pacemaker migrates through the atria and perinodal zone during transesophageal electrical stimulation of the heart (TEC), regardless of the degree of UCTD, the time for recovery of sinus node function and the corrected time for recovery of sinus node function usually do not exceed the physiological norm [4]. This observation suggests preserved automatic activity of the sinus node and the appearance of ectopic complexes due to a change in the rate of diastolic spontaneous depolarization of latent foci of automaticity with an increase in the automaticity of competing centers against the background of unbalanced neurogenic influences. V. M. Yakovlev et al. (2001) suggests a role in the formation of this electrophysiological phenomenon of different sensitivity of the membranes of sinus node cells to acetylcholine and catecholamines. According to Peretolchina T.F. (2000), the frequency of detection of pacemaker migration increases 3 times with increasing severity of the autonomic dysfunction syndrome and the severity of UCTD.
Extrasystole. Atrial and ventricular extrasystole is the most common cardiac arrhythmia among patients with UCTD. The appearance of active ectopic complexes may be associated with changes in the automatic activity of the sinus node, features of innervation, the formation of zones of asynchronous depolarization, activation of atrial latent foci of automaticity due to differences in the rate of repolarization of myocardial fibers and disruption of the electrical homogeneity of the atria, which occurs in conditions of metabolic disturbances in the myocardium of patients with UCTD [ 4].
The frequency of atrial extrasystole varies in patients with UCTD in the presence of mitral valve prolapse (MVP) from 4 to 90% [3]. Atrial extrasystole in patients with UCTD is recorded with various variants of ectopic complexes: more often - with one or more negative spikes in the middle of the complex (type II); somewhat less often - with an initial positive deviation (type I); rarely - with initial and final negative deviation (type III) [4]. The occurrence of supraventricular extrasystoles may be associated with an increase and change in the electrical activity of the cells of the left atrium, which is irritated during systole by a prolapsing myxomatically altered mitral valve leaflet and/or a jet of mitral regurgitation [11]. Thus, according to some observations, significant supraventricular extrasystoles (more than 100 extrasystoles per minute) were recorded only in patients with myxomatous degeneration of prolapsed leaflets [11]. According to our observations, in patients with severe manifestations of UCTD, resting atrial extrasystole increases with physical activity from 10–12 to 18–25 in 1 hour [4].
According to various authors, ventricular extrasystole is observed in 14–89% [3]. According to some data, in patients with echocardiographic signs of myxomatous degeneration of the mitral valve, the average number of ventricular extrasystoles per day and per hour was significantly greater than in the absence of it [11]. Ectopic ventricular activity in patients with UCTD is predominantly represented by ventricular extrasystoles of category I (class I, II according to Lown) and coincides with periods of maximum physical activity [4]. The development of ventricular extrasystole, in addition to autonomic dysfunction and hypersympathicotonia [4, 5, 12], may be associated with abnormal traction of the papillary muscles during MVP [11], mechanical irritation of the endocardium, and myxomatous changes in the chordae [3, 9]. In the genesis of ventricular extrasystoles, the presence of a minor anomaly in the development of the heart - abnormal chords (mechanical irritation of the endocardium at the site of attachment of abnormal chords, the presence of Purkinje cells in the tissues of abnormal chords) may play a role [3]. When the chordal attachment points are located in the area of the interventricular septum or papillary muscles, the risk of potential rhythm disturbances increases [8]. There are observations of a higher frequency of registration of ventricular extrasystoles with a decrease in tissue magnesium content [10].
A small proportion of patients have a combination of atrial and ventricular extrasystoles. “Threatening” extrasystoles are mainly detected in individuals with clear manifestations of DST: stage II–III funnel-shaped deformity, grade II pitched chest deformity [4, 10].
Wolff–Parkinson–White syndrome. In a certain proportion of patients with UCTD, the phenomenon of premature excitation of the ventricles (6.5–8.7–25%) may be detected, due to the functioning of additional impulse pathways [5, 8, 10]. In these patients, in most cases, paroxysmal heart rhythm disturbances are determined in the form of atrioventricular paroxysmal tachycardia.
Paroxysmal tachycardia. In the implementation of the pathophysiological mechanisms of paroxysmal tachycardia in people with UCTD, the participation of dysfunction of the autonomic nervous system with a predominance of vagal influences, additional pathways, and myxomatous changes in the atrioventricular zone is assumed [11]. Paroxysmal rhythm disturbances, according to most researchers, are detected much more often during Holter ECG and TEE compared to recording a resting ECG. Thus, when performing TEES in patients with UCTD, paroxysms of tachycardia are provoked in 72.9% of cases; according to clinical manifestations and ECG configuration, similar to spontaneous previously occurring paroxysms are detected for the first time in 27% of cases. According to the observation of Peretolchina T.F. (2000), paroxysmal tachycardias in patients with UCTD are recorded on a resting ECG in 5.8% of cases, and when conducting a Holter ECG - in 32.5% of observations.
The combination of additional pathways and discrete conduction along the AV junction contributes to the development of paroxysmal supraventricular tachycardias of two types - orthodromic and antidromic. In the first case, in the presence of sinus rhythm and signs of ventricular preexcitation, increased stimulation to a threshold value leads to a return wave of excitation through the accessory bundle of Kent and retrograde excitation of the atria. In another case, antegrade propagation of the impulse occurs through the accessory bundle, and retrograde propagation through the AV junction, which is reflected on the ECG by the appearance of a widened, deformed QRS complex due to the delta wave. The P waves in both cases have an inverted shape, which indicates a retrograde spread of excitation to the atria. According to our observations, the frequency of paroxysms varies from once every 6 months to 3–4 times a week. In this case, attacks occur both during physical activity and at rest or during sleep; poor subjective tolerance of arrhythmia is characteristic. Antidromic reciprocal tachycardia is recorded less frequently and occurs in patients with Wolff–Parkinson–White syndrome.
Paroxysms of ventricular tachycardia are rarely recorded in UCTD - 0.97–2.5% of cases according to various sources, while in all cases there were pronounced manifestations of UCTD with the presence of grades II–III chest deformities [5, 10].
Paroxysmal supraventricular tachycardia, high-grade ventricular extrasystole and paroxysmal ventricular tachycardia recorded during UCTD constitute a threatening basis for the occurrence of fatal rhythm disturbances and sudden death.
Atrial fibrillation/flutter. In general, these rhythm disturbances are rarely recorded - in 3.9–6.2% of cases with CM [5, 8]. The morphological substrate for the electrogenesis of atrial fibrillation/flutter in UCTD can be a genetically determined imperfection in the development of connective tissue in embryo- and ontogenesis and the resulting disruption of intertissue (myocardial and connective tissue structures) interactions, manifested by electromechanical instability; hereditary connective tissue and hemodynamic remodeling of the left (much more often) and right atria; asymmetry of the cellular structures of the right and left atria; transformation of the impulse from the sinus node to the AV junction along the cytological and functional (electrophysiological) structures of the right atrium muscle [11].
Conduction disorders. In most patients, the value of the QRS complex does not exceed the physiological norm (0.10 s). A change in this structural ECG indicator in people with UCTD who do not have organic damage to the heart muscle is a reflection of the bioelectric heterogeneity of the myocardium or an increased load on the outflow tract of the right ventricle. Intraventricular conduction disturbances of various locations are not often recorded (
), having both a transient and permanent character.
More often, these changes are detected against the background of physical activity, less often - at rest [4]. According to O. D. Ostroumova (1995), all patients with right bundle branch block have echocardiographic signs of myxomatous degeneration of the septal leaflet of the tricuspid valve. As is known, it is in this area that the right bundle branch begins, located subendocardially.
Sinoatrial and atrioventricular blocks in patients with UCTD are not often recorded ().
Long QT syndrome. This syndrome as a possible prognostic criterion for sudden death has been described in many studies in patients with MVP. The frequency of its detection varies widely depending on the diagnostic method: when recording a resting ECG - 2.5–26.5%, with a Holter ECG - up to 35.7%, with TEES - up to 42.6% [11] .
Despite the complex multicomponent arrhythmogenesis in each specific clinical observation, the results of numerous studies have noted some general features of the arrhythmic syndrome of UCTD:
- Patients with UCTD often do not have any cardiac complaints, which often only indicates the effectiveness of compensatory mechanisms, but in no case indicates that these individuals have no structural and functional disorders at all. Clinical observations show that it is in such patients that, as a rule, serious cardiovascular lesions are revealed during an in-depth examination [5]. Rhythm disturbances are detected with greater frequency during additional examination - Holter ECG, TEE.
- According to most authors, the incidence of arrhythmias is significantly higher among patients with severe clinical manifestations of UCTD. This indirectly indicates the importance in the formation of rhythm and conduction disturbances of a set of interacting parameters: a combination of extracardial manifestations of connective tissue dysplasia, the severity of metabolic cardiomyopathy, the severity and direction of autonomic dysfunction, the nature and degree of dysplastic-dependent changes in the valve apparatus, the presence of an association with congenital changes in intracardiac architecture (minor developmental anomalies ) etc.
- Most researchers note the maximum number of episodes of cardiac arrhythmias during wakefulness and active activity, while at rest and during sleep their number significantly decreases, which emphasizes the significance of neurogenic influences in the genesis of arrhythmic syndrome in UCTD.
Considering that, according to numerous studies, an unbalanced vegetative background and a decrease in the tissue pool of magnesium occur in the majority of patients with UCTD, correction of arrhythmic syndrome, having features associated with variants of electrogenesis disorders, should include drugs with a vegetotropic effect containing significant macroelements (magnesium). Today it is known that magnesium ions participate in the metabolic processes of connective tissue, control the normal functioning of the cardiomyocyte at all levels of subcellular structures, and take part in the regulation of the contractile function of the myocardium. At the same time, intracellular magnesium deficiency increases the activity of the sinus node, which shortens the atrioventricular conduction time, reduces absolute refractoriness and prolongs relative refractoriness, which can result in the development of various rhythm disturbances. Moreover, the antiarrhythmic effect of magnesium preparations is due not only to the elimination of extracellular electrolyte imbalance and an increase in the intracellular concentration of magnesium and potassium, but also to the activating effect of cations of organic residues on metabolic processes in the myocardium [1].
We observed 120 patients aged from 18 to 42 years (average age 30.30 ± 2.12 years, men - 66, women - 54) with UCTD of varying severity, having chest deformities (funnel deformity of the first degree - 27 people, 22.5%; II degree - 13 people, 10.8%; III degree - 6 people, 5%; keeled (manubriocostal type - 18 patients, 15%; corporocostal type - 19 people, 15.8%; costal type - 12 patients, 10.0%), asthenic form of the chest (7 patients, 5.8%), combined changes in the spinal column - 103 patients, 85.8%); valve syndrome (grade I MVP - 96 people, 80.0%; grade II - 24 people, 20.0%) with or without regurgitation (110 patients, 91.7%)); minor anomaly of cardiac development - abnormal chords of the left ventricle (89 patients, 74.2%); expansion of the root and ascending aorta - 8 people (6.7%).
When questioned, the majority of patients (104 people, 86.7%) had general complaints: weakness, increased fatigue, decreased performance, memory impairment, decreased concentration, dizziness, fainting, headaches, apathy, difficulty falling asleep and/or sensitivity. sleep, restlessness, irritability, feelings of internal tension and/or anxiety. The severity of these complaints, as a rule, increased during periods of intense psychomental stress, significantly affecting work efficiency. In the subjective status, the dominant complaints in terms of frequency of occurrence and severity were complaints from the cardiovascular system: cardialgia (76.7%), sensations of “cardiac discomfort” (23.3%), palpitations (28.3%), interruptions in heart function (16.7%), dizziness, general weakness with a sharp decrease in blood pressure in the orthoptic position (35.0%). Pain in the heart area, as a rule, was stabbing in nature and localized in the apex, or patients noted vague sensations of “cardiac discomfort.” Less frequently, patients noted short-term episodes of palpitations, interruptions in cardiac function, and manifestations of orthostatic hypotension. Quite often, these complaints were accompanied by a feeling of lack of air or a sensation of difficulty breathing.
During the examination (general clinical examination, echocardiography, ECG, Holter-ECG, determination of the level of magnesium in saliva and serum, study of the initial autonomic tone based on clinical tests (A. M. Vein, 1998), assessment of the scale of autonomic disorders of the questionnaire for identifying and assessing neurotic conditions [3]) revealed a syndrome of vegetative-vascular dystonia with a predominance of sympathetic influences (100 people, 83.3%) or vagotonia (5.8%), metabolic cardiomyopathy, arrhythmic syndrome, a significantly lower magnesium content in the oral fluid compared to examined practically healthy volunteers (0.561 mmol/l).
According to the ECG data, all patients revealed changes in the final part of the ventricular complex, which we interpreted in terms of disturbances of metabolic processes in the myocardium within the framework of metabolic cardiomyopathy: I degree of repolarization disturbance (increased amplitude of the T wave V2–4, “TV2 > TV6” syndrome and shortening of the segment ST) was detected in 59 patients (49.2%); II degree of repolarization disturbance (T wave inversion, downward displacement of the ST segment V2–V3 from 0.5 to 1.0 mm) - in 48 patients (40.0%), III degree of repolarization disturbance was determined less frequently - in 10.8%.
Rhythm and conduction disturbances detected during Holter ECG in patients were represented by: sinus tachycardia (76.7%), sinus arrhythmia (23.3%), atrial, ventricular extrasystole (72.5%), AV block I –II degree (28.3%), episodes of supraventricular tachycardia (6.7%), pacemaker migration (4.2%). Heart rate variability in 41 people (34.2%) was increased, in 12 patients (10.0%) it was decreased, and in 67 people (55.8%) it was within normal limits. Rigid rhythm is observed in 7 (5.8%) patients.
All patients were prescribed Magnerot according to the following regimen: 2 tablets 3 times a day for the first 7 days, then 1 tablet 3 times a day for 7 weeks.
After completion of the course of treatment, a statistically significant increase in magnesium content was noted both in the blood serum (from 0.867 mmol/l to 0.955 mmol/l) and in the oral fluid (from 0.561 mmol/l to 0.903 mmol/l). It should be noted that the magnesium content in the blood serum increased faster (after 4 weeks of treatment), while the dynamics of the magnesium content in the oral fluid was reliably detected after completing the full course of taking the Magnerot drug. It is likely that it is the level of magnesium in the oral fluid, as an indicator that most reliably reflects the content of magnesium in tissues, that should be assessed in patients with DST initially, and also monitored during therapeutic interventions.
In general, during treatment, pronounced positive dynamics were revealed in such characteristics of the physical component of quality of life as fatigue (the frequency of symptom detection before and after treatment, respectively - 87.5% (105/120) and 28.3% (34/120), McNemar r2 6.61; p = 0.01) decreased performance (48.3% (58/120) and 6.7% (8/120, respectively), McNemar χ2 39.19; p = 0.000), loss of interest in life (respectively 27.5% (33/120) and 5.0% (6/120) McNemar r2 66.86; p = 0.000). Heart rate variability after treatment was within normal limits in 66.7% (80/120) of patients (initial - 44.2%; McNemar χ2 5.90; p = 0.015). There was a significant change in the autonomic dysfunction scale (-2.35 and 1.28, respectively, before and after treatment), reflecting a decrease in autonomic tension.
Positive dynamics of ECG changes was manifested in a decrease in the incidence of metabolic disorders of repolarization processes of the first degree (McNemar χ2 14.27; p = 0.0002) and second degree (McNemar χ2 10.09; p = 0.002), sinus tachycardia (McNemar r2 12 .69; p = 0.000), sinus arrhythmia (McNemar χ2 4.22; p = 0.04), extrasystole (McNemar r2 9.60; p = 0.002) (
).
During the study, no complaints related to the therapy were noted among the patients.
It should be noted that a decrease in the frequency of detection of the most frequently recorded rhythm disturbances, associated with both a violation of the automatism of the sinus node and the activation of latent foci, was observed against the background of correction of autonomic disorders, improvement of the electrophysiological characteristics of myocardial metabolism and an increase in magnesium content in tissues.
Thus, the drug “Magnerot” for UCTD is well tolerated, reduces autonomic dysregulation, clinical manifestations of metabolic cardiomyopathy, corrects sinus arrhythmias, reduces the frequency of registration of active ectopic complexes, and also has a positive effect on physical performance. The use of complex rehabilitation programs including magnesium preparations (Magnerot) to correct the clinical manifestations of arrhythmic syndrome against the background of metabolic cardiomyopathy and autonomic imbalance is pathogenetically justified and effective.
Literature
- Gromova O. A. Magnesium and pyridoxine: basic knowledge. New technologies for diagnosing and correcting magnesium deficiency / O. A. Gromova. UNESCO training programs, Moscow, RSC Institute of Microelements, UNESCO. 2006. 176 p.
- Mendelevich V.D. Clinical medical psychology: A practical guide. M.: Medpress, 1998. 542 p.
- Domnitskaya T. M. Abnormally located chords. M.: Medical practice. 2007. 95 p.
- Nechaeva G.I. Cardiohemodynamic syndromes in connective tissue dysplasia (clinic, diagnosis, prognosis): Abstract. Dis... Dr. med. Sci. Tomsk 1994. 37p.
- Novak V. G. Clinical and morphological assessment of changes in the cardiovascular system in connective tissue dysplasia in the aspect of sudden death: Abstract of thesis. dis. ...cand. honey. Sci. Tomsk 1997. 16 p.
- Orlov V.N. Guide to electrocardiography. 3rd edition. M.: Medical Information Agency LLC. 2003. 528 p.
- Ostroumova O. D. Echocardiographic and phenotypic features of patients with connective tissue dysplasia syndrome of the heart: Abstract of thesis. dis. ...cand. honey. Sci. M., 1995. 24 p.
- Peretolchina T.F. Mitral valve prolapse and abnormal chordae as a manifestation of connective tissue dysplasia syndrome. Ekaterinburg, 2000. 72 p.
- Stepura O. B. Syndrome of connective tissue dysplasia of the heart: Abstract of thesis. dis. ...doctor. honey. Sci. M., 1995. 48 p.
- Tikhonova O. V. Heart rate variability in young patients with connective tissue dysplasia: Abstract of thesis. dis. ...cand. honey. Sci. Omsk, 2006. 22 p.
- Yakovlev V. M. Rhythm and conduction disturbances in connective tissue dysplasia of the heart / V. M. Yakovlev, R. S. Karpov, Yu. Ya. Belan. Omsk 2001. 160 p.
- Yakovlev V. M. Cardiorespiratory syndromes in connective tissue dysplasia / V. M. Yakovlev, G. I. Nechaev. Omsk: OGMA, 1994. 217 p.
G. I. Nechaeva , Doctor of Medical Sciences, Professor V. M. Yakovlev , Doctor of Medical Sciences, Professor I. V. Druk , Candidate of Medical O. V. Tikhonova , Candidate of Medical Sciences Omsk State , Omsk
Treatment
The main point in the treatment of pacemaker migration through the atria is considered to be the elimination of the cause of the disease. Quite often, after treatment of the primary disease that caused the disorder, the problem disappears.
In addition, general recommendations must be followed:
- get enough rest;
- eat healthy and regularly;
- do physical exercises, such as gymnastics;
- to refuse from bad habits.
In difficult and life-threatening situations, it is necessary to implant a pacemaker (artificial heart pacemaker) to resume stable operation. The main indication for pacemaker installation is hemodynamic impairment, which is characterized by a decrease in blood pressure and progression of symptoms.
Diagnostics
An electrocardiogram is the initial stage of the examination. It provides initial information indicating the occurrence of a problem. If abnormalities are detected, the therapist refers the patient for additional diagnostics. It includes:
- Ultrasound of the heart, liver, adrenal glands, thyroid gland;
- radiography;
- phonocardiography with tone tracking;
- blood test for hemoglobin, cholesterol.
The most informative diagnostic method is Holter monitoring. During the day, the patient walks with a connected device that records heart function, rhythm, and shows deviations during sleep and walking. If necessary, a person receives consultation from an endocrinologist, cardiac surgeon, or neurologist.