Changes in biochemical parameters
During pregnancy, a decrease in the total concentration of protein in the blood plasma (albumin) is due to partial dilution of the blood due to an increase in its total volume. But it can occur as a result of fluid retention in the body, hemodynamic disturbances, and increased vascular permeability during pregnancy.
Changes in the concentration of blood proteins are detected on the proteinogram. In the first and second trimester of pregnancy, albumin decreases. In the third trimester, an increase in the alpha-1-globulin fraction, alpha-fetoprotein, is detected.
The alpha-2-globulin fraction can increase due to proteins associated with pregnancy (begin to increase from 8-12 weeks of pregnancy and reach a maximum in the third trimester). Betta and gamma globulins also increase. Minor changes in C-reactive protein, observed more often in the early stages of pregnancy, may be the body’s reaction to the processes of increased cell division during the growth and development of the baby.
Changes in circulating blood volume (CBV) and blood supply to the kidneys lead to changes in the excretory function of the kidneys. There is a delay and accumulation of nitrogenous substances, while the amount of urea decreases, especially in late pregnancy. Creatinine levels decrease maximally in the 1st-2nd trimester (its concentration can decrease by almost 1.5 times), which is associated with muscle growth in the mass of the uterus and child. The level of uric acid is often reduced due to increased blood supply to the kidneys. But even minor disturbances in kidney function can lead to an increase in this indicator, and this is regarded as a possible symptom of intoxication.
Fat metabolism changes significantly during pregnancy. Since metabolic processes in the body intensify, cholesterol levels (cholesterol, high-density lipoprotein HDL) increase.
An increase in the level of estrogen hormones during pregnancy leads to the deposition of fat in the mammary glands, waist and buttocks. Therefore, losing weight and going on diets is useless for pregnant women. This is hormonal and will go away only after the child is one year old, almost regardless of whether the mother breastfeeds or not.
During pregnancy, insulin levels increase. The indicator reflecting the level of insulin is C-peptide. In this case, the glucose level may change slightly or not change at all.
However, glucose can be detected in urine during normal pregnancy. This happens because during pregnancy the rate at which urine is filtered through the kidneys increases. Most often, glucose in the urine appears during pregnancy 27-36 weeks.
Features of mineral metabolism in healthy pregnant women compared to non-pregnant women is the retention of sodium, potassium, chlorine, and phosphorus salts in the body. And it is precisely the changes in phosphorus levels in the pregnant woman’s body that are associated with an increase in alkaline phosphatase. This is due to changes during pregnancy in bone tissue and changes in the liver.
As you know, during pregnancy the need for calcium salts, which are necessary for the formation of the baby’s skeleton, increases. Therefore, the mother may experience calcium deficiency, which sometimes manifests itself in muscle cramps.
Increased iron consumption by the body of a pregnant woman can lead to anemia. This condition is characterized by a decrease in iron, ferritin, vitamins: B12, folic acid.
Complications after gestosis
The last stage of pathology can lead to a number of serious consequences:
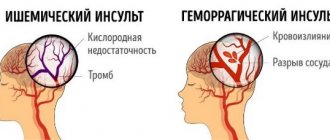
- Intracerebral hemorrhage.
- Hemorrhagic shock.
- Premature placental abruption.
- Retinal detachment and vision loss.
- Acute renal failure.
- Coma after convulsions.
Fetal complications:
- Developmental delay syndrome.
- Intrauterine oxygen deficiency (hypoxia).
- Antenatal death.
Such conditions can be avoided by undergoing regular examination and observation by an experienced gynecologist.
Causes of pathology
There are several theories that explain the development of preeclampsia and eclampsia in pregnant women. Let's look at the main ones:
- Cortico-visceral theory. According to her, gestosis is a neurotic condition that occurs due to a disruption of the physiological connection between the cortex and subcortex of the brain.
- Immunological. Pathology occurs due to an incorrect reaction of the pregnant woman’s immune system to fetal antigens.
- Genetic. There is speculation about a possible “eclampsia gene” that is inherited. According to the theory, gestosis is 8 times more common in women whose mothers suffered from a similar disease.
- Endocrine. According to this theory, pathology develops due to improper functioning of the body's endocrine system.
- Placental. The disease is caused by impaired development of the placenta, as a result of which blood flow is disrupted and hypoxia occurs.
It is worth noting that a unified theory explaining the development of gestosis has not yet been identified. According to scientists, a combination of several factors leads to pathology.
Women who have one of the following factors are at increased risk:
- Age under 18 and over 35 years old.
- Multiple pregnancy.
- Postponed early gestosis.
- Extragenital diseases.
- Presence of obesity.
Symptoms of the disease
The most obvious signs of pathology are the following:
- Constant headaches, poor health.
- "Floaters" before the eyes.
- Nausea and vomiting, fever.
- Dry cough, hoarseness of voice.
- Chills, fever.
- Swelling of the legs, arms and face.
- Arterial hypertension.
If such symptoms appear in the 3rd trimester, a woman is prescribed a number of additional examinations to diagnose gestosis. Most often, the patient additionally exhibits disturbances in uteroplacental blood flow, the presence of protein in the urine, a decrease in the number of platelets and a decrease in the concentration of anticoagulants.
Treatment and prevention
To diagnose the early stages of gestosis, the McClure-Aldrich test is used, which allows you to identify hidden edema. A blood test is also performed to determine the platelet count, as well as the presence of protein. A Doppler examination of the fetus and an ultrasound of the kidneys are performed. In our clinic, Professor S.M. Voevodin and Professor A.D. Lipman conduct an expert study - ECG + blood flow in the uterine and fetal vessels (ultrasound-EKIPFS). This study is done using specialized equipment and allows us to identify the risks of gestosis at a preclinical level or assess the dynamics of fetal development, as well as the effectiveness of therapy against the background of gestosis. In addition, a scientific group of clinic doctors has developed an algorithm for managing a patient at risk of preeclampsia and miscarriage, which allows minimizing risks.
Based on the diagnostic results, the patient’s health status is determined. If signs of gestosis are detected, a number of drugs are prescribed, including sedatives and antihypertensives, anticoagulants and antioxidants. Intravenous influences can be carried out aimed at replenishing the volume of circulating blood. The fact is that in most patients with gestosis, the hemostasis system is disrupted. Therefore, consultation or observation with a hemostasiologist is important. If treatment does not bring the desired results, then delivery is necessary - most often a cesarean section is performed.
But it is worth noting that these are extreme measures; with a competent approach from a specialist, it is possible to detect and treat gestosis during pregnancy in the early stages. Therefore, be sure to tell your doctor even if you feel a slight deterioration in your health.
If you have any questions about this pathology, you can ask them to the specialists at our International Hemostasis Clinic in Moscow. Make an appointment using the online form or by phone.
Blood clotting during pregnancy
The blood coagulation system is one of the most complexly regulated systems in our body. The processes of thrombus formation are in a certain balance with the processes of fibrinolysis. If this balance is disturbed, pathological phenomena can be observed - hypercoagulability or, on the contrary, a tendency to bleeding. Of course, this is always facilitated by certain factors, one of which is the pregnancy period.
During pregnancy, under the influence of various influences, most normal processes in the body change. The most well-known fact is the decrease in immunity, which is considered as a protective reaction of the body. After all, the fetus in the early stages can be perceived as a foreign protein, in which case the insufficient functioning of the immune system is only for the good, since the body does not fight against the baby. But not everyone knows about this feature and in some cases they try by any means to influence the state of immunity in order to avoid frequent viral diseases and exacerbation of existing chronic infections.
Similar changes occur with the blood coagulation system. During pregnancy, increased thrombus formation normally occurs. This can be explained by the body’s preparation for subsequent possible blood loss during childbirth. Also, the properties of blood during pregnancy change due to the increasing load - blood flow and sufficient nutrition are required towards the placenta, which is abundantly supplied with blood. The developing systems and organs of the fetus also affect the characteristics of blood clotting. If such changes are insignificant and fit within the norms for pregnant women, then pathology of hemostasis is excluded. But in cases where there are pronounced disorders, especially those accompanied by a clinical picture, it is important to diagnose this condition in a timely manner and decide on the need for drug correction.
One of the indicators that pay special attention to during pregnancy is D-dimer.
. D-dimer is a breakdown product of a formed blood clot, thrombus. The more pronounced thrombus formation, the more D-dimers are formed from blood clots. Based on their level in the blood, one can assume hypercoagulation, that is, increased coagulation and thrombus formation.
During pregnancy, D-dimer is normally slightly elevated
. Please note that laboratories always provide reference values by trimester, but only the attending physician can accurately diagnose pathological coagulation, taking into account complaints and the clinical picture. First of all, the obstetric and gynecological history is taken into account. Thus, in case of previously unsuccessful pregnancies that ended in miscarriage, loss of the fetus, or if an elevated D-dimer is detected, a consultation with a hematologist is indicated to resolve the issue of prescribing anticoagulants. But if an increase in D-dimer is observed once, and there are no signs of thrombus formation, the patient’s medical history is unremarkable, doctors usually choose observational tactics, although several years ago even slight increases in D-dimer forced specialists to include heparins in therapy. Now clinical recommendations are drawn up in such a way that the approach to studying the properties of blood is extremely comprehensive and is not limited to just the assessment of D-dimer. Be sure to take into account the general coagulogram, the presence of varicose veins, a history of thrombosis, and the presence of recurrent miscarriage syndrome.
In women with 2 or more pregnancy losses, an in-depth study of blood properties is indicated already at the planning stage. It should be noted that against the background of existing pathological blood conditions, conception is quite possible, but further progression of pregnancy without proper correction can be significantly difficult and even impossible.
The most common problems in the 1st trimester of pregnancy
Bleeding : due to a lack of progesterone, spasm and relaxation of the muscles of the uterus occurs and, accordingly, detachment of the ovum and bleeding (in severe cases, miscarriage). Also, with hypothyroidism, there is a violation of the implantation of the fertilized egg, which also leads to bleeding.
Thrombosis . During pregnancy, blood clotting increases (this is normal - the body is preparing for blood loss). To prevent thrombosis of the veins of the lower extremities, it is necessary to stop taking oral contraceptives 6 months before planning a pregnancy, and during pregnancy, maintain sufficient physical activity (long walks, swimming) and be observed by a doctor. If pain, swelling, or “cyanosis” occurs in the lower extremities, you should immediately consult a doctor.
Developmental anomalies and genetic pathologies . In the 1st trimester, abnormalities in the development of the fetus most often develop and manifest themselves, which can occur as a result of ionizing radiation, infectious diseases suffered during pregnancy (rubella, influenza, etc.), taking drugs prohibited during pregnancy (fluoroquinolones, macrolides, tetracyclines, tranquilizers , etc.), genetic pathologies and hereditary diseases.
Anemia . More often it happens due to iron deficiency, because. the fetus consumes a huge amount of it, taking away iron reserves from the mother, less often due to bleeding. B12 and folate deficiency anemia also occur, which is also associated with increased consumption and insufficient consumption by the mother. Anemia is dangerous due to chronic lack of oxygen, causing fetal growth restriction syndrome. In severe cases, it can lead to intrauterine fetal death.
Chronic diseases may worsen during pregnancy . This is due to the fact that the fertilized egg already carries part of the DNA that is foreign to the mother. To prevent egg rejection, the mother’s body suppresses its immunity throughout pregnancy, which affects the body as a whole. Therefore, all diseases that were not detected at the stage of preparation for pregnancy should be detected and treated. This applies mainly to infectious diseases. For example, exacerbation of chronic pyelonephritis, or herpes virus infection.
Also, during pregnancy there is a sharp restructuring of hormonal levels , which, in turn, affects somatic (non-infectious) diseases. For example, due to thickening of bile and compression of the gallbladder by the uterus, chronic cholecystitis may worsen. As the fetus develops, the islets of Langerhans (cells that produce insulin in the pancreas) sometimes do not develop quickly enough and the baby begins to consume the mother's insulin. If the mother has diabetes or impaired glucose tolerance (including hidden), both the child and the mother lack insulin, which leads to tissue damage to both the child and the mother (early aging of the placenta, delayed fetal development, premature birth, polyhydramnios, increased blood pressure in a pregnant woman, the formation of a large fetus, the risk of injury to the woman and child during childbirth and the most dangerous complication - intrauterine fetal death).
In addition, the fetal liver does not yet function as a detoxification organ, and all decay products produced by the child are forced to be processed by the mother’s liver. 2% of pregnant women develop benign cholestatic hepatosis of pregnancy - a violation of the formation and outflow of bile, which causes:
- Itchy skin, especially at night
- Digestive disorders (as bile is involved in the emulsification of fats)
- Inflammation of liver tissue due to toxic effects on cells and bile ducts
Classification of pathology
This disease is often also called late toxicosis, but this name is incorrect. Unlike toxicosis, gestosis requires treatment, since it is not a normal variant. Depending on the severity of symptoms, there are 4 stages of the disease:
- Dropsy. It often goes unnoticed, as it is characterized only by swelling.
- Nephropathy. In addition to swelling, a pregnant woman may find protein in her urine. At this stage, kidney damage occurs; if left untreated, complications may arise.
- Preeclampsia. Characterized by increased blood pressure and accompanying symptoms: headache, nausea, etc. Possible disruption of cerebral blood supply.
- Eclampsia. This stage is the most dangerous. A pregnant woman often experiences convulsions, placental abruption, heart attack and other serious consequences can occur.
The discovery of new forms of thrombophilia, the role of hypercoagulability disorders in the onset and progression of various complications of pregnancy determine the importance of studying this problem. Currently, simple coagulogram indicators are not enough to objectively assess the function of the hemostatic system. Laboratory studies that identify markers of thrombophilia make it possible to timely diagnose hypercoagulable states and determine treatment options for such patients. Thrombophilia is the tendency of the body to form hypercoagulable states, caused by a violation of the regulatory mechanisms of the hemostatic system or changes in the properties of individual parts of this system. Z.S. Barkagan and A.P. Momot [1] define thrombophilia as “disorders of hemostasis and hemorheology, which are characterized by an increased tendency to develop thrombosis of blood vessels and organ ischemia, which are based on disturbances in various parts of the hemostatic system.” In the last 20 years, much attention has been paid to the study of thrombophilias. This is due to major advances in the field of hemostasiology (diagnosis of hypercoagulability disorders and their correction) and the widespread prevalence of thrombophilic conditions. In 1965, researcher O. Egeberg [2] first described the pathology, which consisted of a tendency to develop thrombosis at a young age, associated with a decrease in the level of antithrombin III (AT III). Subsequently, other possibilities for the development of thrombophilia were proven: changes in the structure of the AT III molecule, protein C deficiency (J. Griffin et al. [3]), protein S defect [4]. In 1993, B. Dahlback et al. [5] described resistance to activated protein C—activated protein C resistance, or factor V Leiden disease.
The identification of mutated prothrombin 20210 A, leading to an increase in its content in the blood by almost 25%, made it possible to discuss the issue of a new class of thrombophilias that arise due to an excess of procoagulants in the blood [6, 7]. Significant progress in understanding the development of the human body’s increased susceptibility to thrombosis has been the discovery of a connection between the frequency of thrombosis and the level of homocysteine in the blood [8]. With the development of diagnostics of the causes of thrombophilia and the establishment of new etiological factors, the frequency of detection of pathology also increased. For a genetic defect in thrombophilia in chronological order in persons with venous thrombosis, this indicator was as follows: before 1965 - 0, 1965 (the value of antithrombin was discovered) - less than 5%, 1981 (the value of protein C was discovered) - less than 10 %, 1984 (the value of protein S was discovered) - about 10-12%, 1994 (the value of APC resistance was discovered) - about 60%, 1996 (the value of prothrombin 20210A) - about 80% [6, 7] . Currently, the following factors are of greatest clinical importance: 1) factor V Leiden; 2) prothrombin mutation 20210A; 3) AT III; 4) protein S defect; 5) protein C defect; 6) hyperhomocysteinemia. In the absence of genetic abnormalities, the components of the hemostasis system maintain the blood in a normal state. Blood coagulation occurs through the interaction of the vascular-cellular and plasma components of this system. Blood coagulation is controlled by the action of anticoagulant proteins. AT III is a plasma protein that reduces the activity of serine proteases of the internal and general coagulation pathways. In the presence of endogenous heparan sulfate, the rate of their inactivation increases. Plasma cofactors - factors VIII and V - are inactivated when they are cleaved by protein C, which is activated by thrombin in the presence of thrombomodulin associated with endothelial cells. In the presence of protein S, which acts as a cofactor, the rate of activation increases significantly. An inhibitor of the extrinsic coagulation pathway is a lipoprotein-associated plasma protein. It participates in the formation of a complex with tissue factor and activated factors VII and X, which leads to their inactivation. The formed thrombus is affected by plasminserin protease, which is formed as a result of enzymatic reactions from plasminogen.
APC resistance is the most common cause of thrombophilia. It is determined in the population in 20% of patients with the onset of thrombosis, in 50% - with hereditary disorders of the hemostasis system, and in 60% - with thrombosis with normal levels of proteins C, S, AT III. Patients who exhibit APC resistance have a point mutation in the coagulation factor V gene (Leiden mutation). With this mutation, the clotting factor becomes resistant to the cleavage action of activated protein C. The heterozygous Leiden mutation is detected in 5% of patients and leads to a 3-7-fold increase in the risk of blood clots. The homozygous form of this mutation increases the risk of thrombosis by approximately 80 times [9–11]. The likelihood of thrombosis increases when the Leiden mutation is combined with other disorders of the hemostatic system - protein S deficiency, hyperhomocysteinemia and pregnancy. The Leiden mutation is determined using DNA diagnostics (polymerase chain reaction - PCR). Diagnosis of APC resistance is carried out using coagulation tests over time.
Protein C deficiency is detected in no more than 0.5% of the general population. In patients with the onset of thrombosis, it is found in no more than 3% of cases [12, 13]. In the heterozygous form of this mutation, the risk of developing thrombosis increases 7 times [14]. In families whose members have this mutation, the incidence of thrombosis is approximately 50%. The level of protein C in the blood in this form is determined in the range from 35 to 65% of standard values. The onset of thrombosis in people with this mutation occurs between the ages of 10 and 50 years. Newborns with homozygous protein C deficiency may develop purpura fulminans or disseminated intravascular coagulation. These conditions were incompatible with life until fresh frozen plasma, a source of protein C, was used. More than 160 mutations can be the causes of protein C deficiency [15]. Protein C deficiency occurs in two types: quantitative (type 1) and qualitative (type 2). Type 1 is characterized by the presence of a reduced amount of normal protein C. In type 2, the blood contains a large amount of protein C with low activity. The functional activity of protein C is determined using coagulological research methods, and its level is determined by the enzyme immunoassay method.
Protein S, a cofactor in the inactivation reaction of coagulation factors Va and VIIIa, consists of free protein S (40%) and bound protein (60%) with C4B-binding protein. Hereditary protein S deficiency is found in 0.7% of people in the general population and 3% of patients with venous thrombosis. In families with this pathology, the incidence of thrombosis is 19-47% at a young age (10-50 years). The risk of thrombosis increases when associated with other hereditary thrombophilias. Inherited protein S deficiency can be caused by more than 70 mutations in the gene encoding the synthesis of this protein [13, 16]. The types of deficiency are the same as for protein C deficiency.
AT III is a powerful natural anticoagulant that, along with thrombin, inhibits several other coagulation factors - activated factors IX, X, XI and XII. Deficiency of A.T. III in the general population is detected in 0.17% of cases, among patients with thrombosis and pulmonary embolism - in 1.1%. In families with hereditary AT III deficiency, thrombotic complications occur in 50% of relatives. In individuals heterozygous for AT III deficiency, its level is 45-75%. The highest incidence of thrombosis in this form of thrombophilia occurs between the ages of 15 and 35 years. In general, the risk of thrombosis caused by AT III deficiency exceeds that with a deficiency of proteins C, S and APC resistance. Homozygous deficiency of AT III is not compatible with life, with the exception of a deficiency associated with a defect in the heparin-binding domain of the AT III molecule as a result of a corresponding mutation. Patients with this type of deficiency have a high risk of not only venous, but also arterial thrombosis [17].
The mutation of the prothrombin gene 20210A is determined by a constantly high level of prothrombin in the blood plasma (in 87% of carriers it exceeds 115%). The mutation is inherited in an autosomal dominant manner, its heterozygous form occurs in 2-3% of people in the general population and 6.2% of patients with venous thrombosis [13]. The G20210A mutation is associated with a high risk of thrombosis not only in peripheral veins and veins of the brain, but also in arteries with the development of ischemic strokes and coronary heart disease in young patients.
Hyperhomocysteinemia occurs in the population with a frequency of 5-10% and is defined as mild when the level of homocysteine in the blood plasma is 15-30 µmol/l, moderate - at a level of 30-100 µmol/l and severe - at a level of more than 100 µmol/l. The frequency of severe hyperhomocysteinemia in the population is 0.4%. Hyperhomocysteinemia is detected in 10-25% of patients with venous thrombosis [18, 19]. It is also an independent risk factor for the development of atherosclerosis.
The homozygous form of cystathione-β-synthase deficiency (hereditary homocysteinuria) is rare - affecting 1 in 200,000 newborns [13]. It is characterized by extremely high levels of homocysteine in the blood, often exceeding 400 µmol/l, and is clinically manifested by the early development of venous and arterial thrombosis, as well as atherosclerosis in individuals with skeletal pathology and mental retardation. Heterozygous deficiency of cystathione-β-synthase is characterized by moderate hyperhomocysteinemia, usually not exceeding 20-40 mmol/l, and does not appear until the first episode of venous or arterial thrombosis at a young age. The frequency of this form of hyperhomocysteinemia in the population is 0.3–1.4% [20, 21].
Deficiency of 5,10-methylenetetrahydrofolate reductase is much more common - in 5% of people in the general population (15% in the USA and Canada). In patients with atherosclerosis, this form of hyperhomocysteinemia occurs in 19% of cases [22, 23].
The mechanisms of prothrombogenic and anti-atherosclerotic effects of homocysteine include damage to endothelial cells with subsequent activation of platelets and expression of tissue factor that activates the coagulation cascade, lipid peroxidation, oxidative modification of low-density lipoproteins, which enhance damage to the vascular wall [24]. The combination of hyperhomocysteinemia with other forms of thrombophilia increases the risk of developing thrombosis.
Increased homocysteine levels can be easily eliminated by taking vitamins B12, B6 and folic acid. However, it is still unclear whether its normalization leads to a reduction in the risk of developing venous and arterial thrombosis, although studies demonstrating such a connection have appeared [25].
Pregnancy is always accompanied by a state of hypercoagulability, which is associated with increased levels of fibrinogen and prothrombin; also significantly by 50-80% - the level of blood clotting factors VIII, IX, X increases. At the same time, the activity of the fibrinolysis system and physiological anticoagulants decreases: the activity of the plasminogen activator inhibitor increases with a simultaneous increase in the level of plasminogen activators - t-PA, u-PA, FXII. As the duration of pregnancy increases, the speed of blood flow in the veins of the lower extremities decreases, due to compression of the inferior vena cava by the pregnant uterus, which additionally creates conditions for the formation of blood clots in the lumen of the veins. The incidence of venous thrombosis and thromboembolic complications during pregnancy is 0.7–1.3 per 1000, which is almost 10 times higher than among non-pregnant women of fertile age [9]. In addition, against the background of thrombophilia, changes in the placenta also occur: instability of the hemostatic balance in the mother-placenta system occurs during a complicated pregnancy, when, under the influence of a number of factors, numerous damage to the epithelial cover of the placental villi appears, leading to disruption of the brush border, exposure of the basal layer and even villous stroma and the release of additional placental coagulating factors [27]. The latter is accompanied by activation of the external coagulation system, and subsequently triggers the internal pathway of coagulation of maternal blood in the intervillous space.
An important discovery of the 20th century was the recognition of the role of thrombophilia in the development of complications such as recurrent miscarriage, fetal growth restriction syndrome, premature placental abruption, non-developing pregnancy, and antenatal fetal death [28, 29]. If previously the causes of antenatal losses were mainly considered to be chromosomal, anatomical, endocrine, infectious and immune factors, now disorders in the blood coagulation system—hereditary and acquired defects of hemostasis—are identified as a separate group of causes of reproductive losses [30]. The frequency of spontaneous miscarriages in Russia is about 15–20% among all wanted pregnancies, reaching 40–50% [31] in the first trimester, and remains stable primarily due to the multifactorial nature of this problem [32, 33].
Fetal loss syndrome (FLS) is a broad concept and includes: the presence of one or more spontaneous miscarriages at 10 weeks or more of pregnancy; stillbirth; death of a premature fetus as a complication of premature birth, severe gestosis or placental insufficiency; 3 or more spontaneous miscarriages at the preembryonic or early embryonic stage, including unsuccessful attempts at in vitro fertilization [40].
One of the other significant causes of this pathology is antiphospholipid syndrome (APS). It can be either sporadic or hereditary. The development of APS is associated with carriage of the DR 4, DR 7, DRw 53, DRB 1 loci of the HLA system [35, 36]. Phospholipids of cell membranes take an active part in the implantation process. Normally, negatively charged phospholipids on the surface of cells are a signal for cell destruction by macrophages and local activation of blood clotting. Trophoblast is the only tissue whose cells exhibit negatively charged phospholipids on their surface for a long time. The negatively charged phosphatidylserine of the trophoblast is coated with a natural anticoagulant, annexin V. Annexin V, also known as placental anticoagulant protein (PAP I), belongs to a family of calcium-dependent phospholipid-binding proteins. The affinity of annexin V for phospholipids is 1000 times higher than that of prothrombin. The pathogenesis of APS is based on the process of thrombus formation, induced by the interaction of antiphospholipid antibodies (APA) with phospholipids of platelet membranes, endothelium and phospholipid-bound plasma proteins. The main fraction of AFA are anticardiolipin antibodies (ACA). These antibodies are found in various autoimmune diseases. The number of cases of detection of AKA in women with recurrent miscarriage in Russia is 28-31%. Antibodies to b2-GP 1 can belong to classes G, M and A [37]. AFA directly interact with syncytiotrophoblast and cytotrophoblast and inhibit intercellular fusion of trophoblast cells. Among the AFAs, the most clinically significant are lupus anticoagulant (LA), antibodies to cofactor b2-GP 1, antithrombin antibodies and antibodies to annexin V. The damaging effects of APAs can occur in several ways: the adhesive characteristics of the preimplantation embryo change; syncytium fusion is disrupted; the depth of trophoblast invasion decreases; the production of human chorionic gonadotropin is suppressed; thrombotic tendencies are enhanced by providing templates for coagulation reactions. The latter factor explains the positive effect of anticoagulant therapy from the earliest stages of pregnancy. These mechanisms also help explain unsuccessful attempts at artificial insemination and embryo transfer in women with AFA. Circulation of AFA is more often found in patients with early miscarriages - in 43.1% [38], with late miscarriages - in 22.4% and with early preembryonic losses - in 35.7% [39]. The risk of fetal loss in women with VA and/or APA can reach 28%, in contrast to 7% in the general population [40].
The incidence of premature abruption of the normally located placenta (PNAP) tends to increase and currently amounts to 0.3-0.4% of all births.
Placental abruption is a manifestation of a systemic, sometimes hidden pathology in pregnant women. Changes in hemostasis are the cause and consequence of PONRP. In the development of PONRP, great importance is attached to APS, genetic defects of hemostasis described earlier, predisposing to thrombosis. Thrombophilia, which develops as a result of these disorders, prevents full trophoblast invasion, contributing to placentation defects [41].
Hemostasis disorders can occur as a result of PONRP, for example, the acute form of DIC [42], leading to massive bleeding and the development of PONRP. The situation is typical for central abruption, when pressure increases in the area of blood accumulation, conditions arise for the penetration of placental tissue cells with thromboplastic properties into the maternal bloodstream.
Fetoplacental insufficiency is a symptom complex in which various disorders occur in both the placenta and the fetus. This pathology has a wide range of disorders that depend on the duration of pregnancy, the strength, duration and nature of the impact of the damaging factor, as well as on the compensatory capabilities of the mother-placenta-fetus system. This problem is multifactorial, and one of these factors is hypercoagulability disorders, the manifestation of which is impaired nutritional function and, as a consequence, the development of fetal growth restriction syndrome [43].
The role of hypercoagulability disorders in the course and progression of many diseases determines the importance of studying this problem, especially in the field of obstetrics and gynecology. Many aspects of this problem have been described and studied, every year many scientific articles and clinical case studies bring us closer to a full understanding of this problem, however, the risks of an unfavorable pregnancy outcome with insufficient attention to this pathology are very high, therefore, timely diagnosis and correctly prescribed treatment can save many lives and families.
The authors declare no conflict of interest.
Authors declare lack of the conflicts of interests.
*e-mail; https://orcid.org/0000-0001-8117-9054